Figure 1. Key Steps of Reverse Cholesterol Transport.
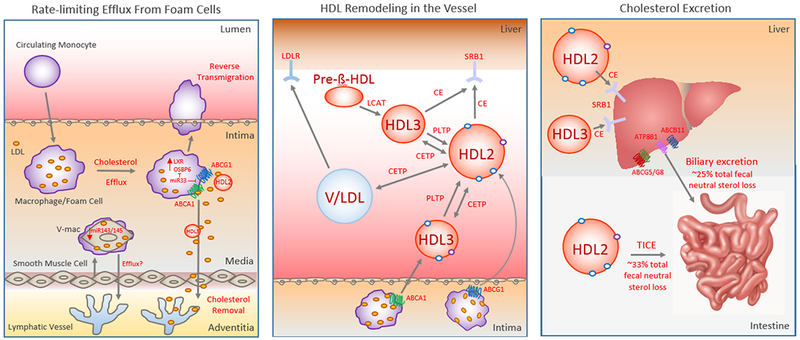
Reverse Cholesterol Transport (RCT) begins with the removal of cholesterol from arterial foam cells that are of vascular smooth muscle cell (V-mac) or macrophage origin (left panel). This is the rate-limiting step of the RCT pathway, and requires the efflux of free cholesterol to cholesterol acceptors such as nascent or mature HDL along with macrophage egress from the plaques. While RCT from macrophage foam cells requires the cholesterol pumps ABCA1 and ABCG1, mechanisms regulating RCT from intimal vascular smooth muscle cells that have transdifferentiated to macrophage-like foam cells (V-mac) are not well understood, though cholesterol efflux from V-macs appears defective relative to macrophage foam cells. Intimal-derived HDL cholesterol can reach the liver 1. directly through binding the hepatic HDL receptor SR-B1 that selectively removes CE from HDL2 and HDL3 or 2. indirectly via apoB-containing lipoproteins (VLDL or LDL [V/LDL])-- to which cholesterol is transferred by the action of cholesterol ester transfer protein (CETP)– that are cleared by hepatic LDLR (middle panel). Phospholipid Transfer Protein (PLTP) also plays an important role in regulating HDL metabolism through HDL remodeling. Finally, the last step of the RCT pathway is cholesterol excretion into the feces (right panel). This can occur through biliary cholesterol excretion or trans-intestinal cholesterol efflux (TICE) that mediate approximately 25% and 33% of total fecal neutral sterol loss, respectively.
