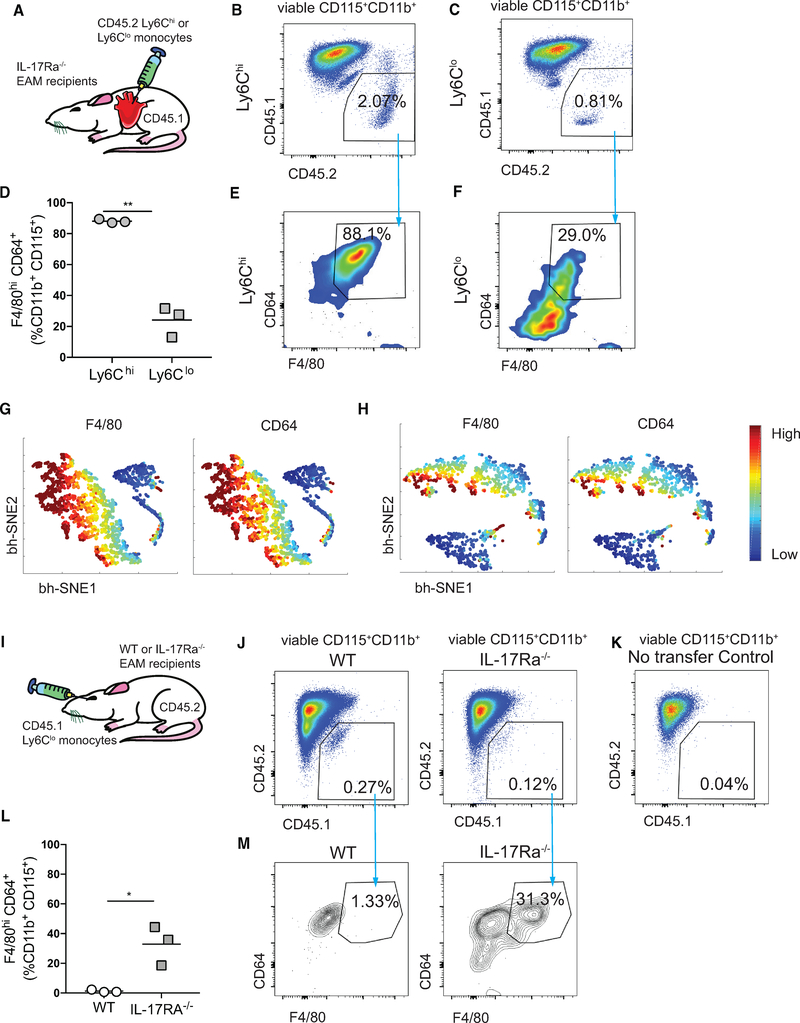
Figure 5. The Absence of IL-17A Signaling Enables Ly6Clo Monocyte-to-Macrophage Differentiation In vivo.
(A) Schematics of intracardiac injection of CD45.2+Ly6Chi or CD45.2+Ly6Clo monocytes into CD45.1 day 21 EAM IL-17Ra−/− recipient mice.
(B and C) Gating of concatenated Ly6Chi (B) and Ly6Clo (C) donor cells from the total viable CD115+CD11b+ population.
(D) Percentages of injected Ly6Chi or Ly6Clo MDMs.
(E and F) Frequencies of Ly6Chi MDMs (E) and Ly6Clo MDMs (F) out of the viable CD45.2+CD115+CD11b+ population.
(G and H) F4/80 and CD64 expression intensities of Ly6Chi (G) and Ly6Clo (H) MDMs using bh-SNE-dimensional reduction algorithm.
(I) Schematics of retro-orbital injection of CD45.1+Ly6Clo monocytes into CD45.2 day 21 EAM WT or IL-17Ra−/− recipient mice.
(J) Gating of concatenated Ly6Clo donor cells from the total viable CD115+CD11b+ population in WT and in IL-17Ra−/− recipient ice.
(K) CD45.1+ monocytes were gated based on no injection control CD45.2+ mice.
(L) Percentages of CD45.1+ Ly6Clo MDMs in the heart.
(M) Frequencies of Ly6Clo MDMs out of the viable CD45.1+CD115+CD11b+ population in WT and in IL-17Ra−/− recipient mice. Data are representative of two independent experiments with biological triplicates.
(D and L) n = 3. Groups were compared using Student’s t test. *p < 0.05, **p < 0.01. All data were presented as mean ± SD.
See also Figure S5.