Fig. 2: Full-length IL-33 stored in the ECM is protected from proteolytic degradation by incorporation into the lumen of MBV.
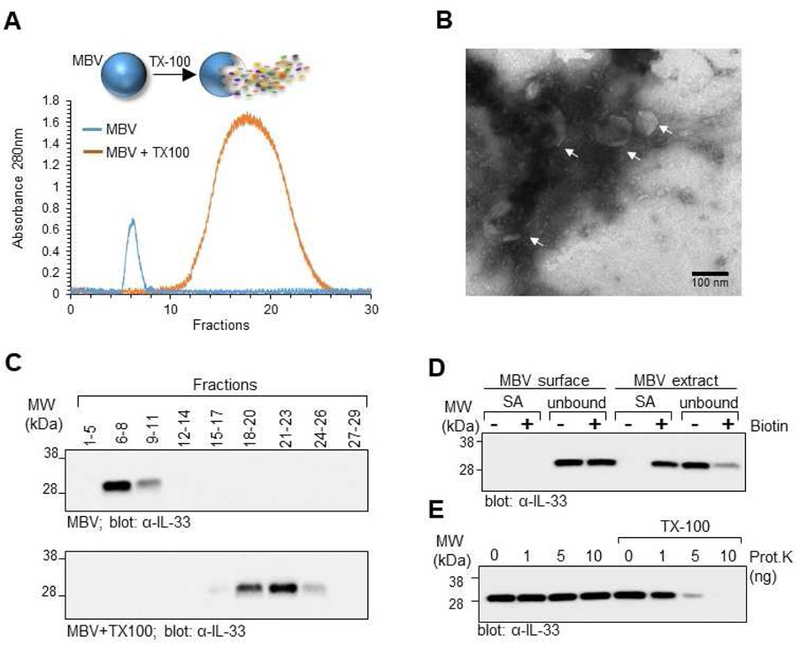
(A) MBV isolated from decellularized wt mouse intestines were fractionated by size exclusion chromatography (SEC) with continuous monitoring of eluted fractions by UV absorbance at 280nm. In a separate experiment, MBV were first lysed with Triton X-100 and then fractioned by SEC. Overlay of the two UV chromatograms shows that intact MBV eluted in the heavier fractions, whereas the molecular components of lysed MBV eluted primarily in the lighter fractions. (B) Pooled fractions 6–8 of the intact MBV sample were imaged by transmission electron microscopy to confirm the presence of MBV in the pooled fraction. (C) Eluted fractions from the intact MBV sample (top panel) or lysed MBV (bottom panel) were pooled as indicated and analyzed by immunoblot for IL-33. (D) Pooled fractions 6–8 of intact MBV were either directly biotinylated to label the MBV surface proteins, or first lysed with Triton X-100 and the MBV extract biotinylated to label the luminal and surface proteins. Proteins isolated after streptavidin pull down (SA) and the unbound fraction representing proteins that did not bind to the streptavidin beads (unbound) were analyzed by immunoblot for the presence of IL-33. (E) Proteinase K protection assay. Pooled fractions 6–8 of intact MBV were treated with indicated amounts of Proteinase K in the absence or presence of Triton X-100 and analyzed by immunoblot for IL-33.
