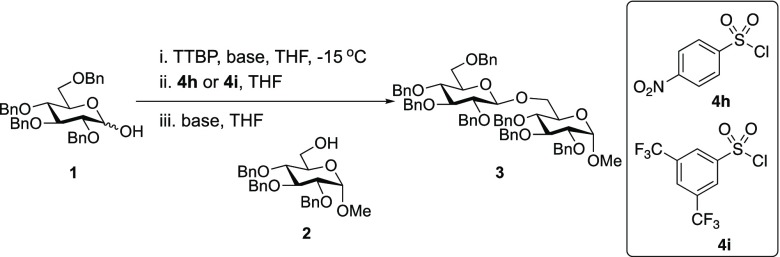
Table 2. Optimization of Glycosylation Conditionsa.
| entry | base | sulfonylating agent | yield (%)b | β/α ratioc |
|---|---|---|---|---|
| 1 | KN(SiMe3)2 | 4i | 46 | β only |
| 2 | LiN(SiMe3)2 | 4i | NR | NR |
| 3 | NaN(SiMe3)2 | 4i | 69 | β only |
| 4d | NaN(SiMe3)2 | 4i | 81 | β only |
| 5d,e | NaN(SiMe3)2 | 4i | 96 | β only |
| 6d,e | NaN(SiMe3)2 | 4h | 85 | β only |
0.20 mmol of glucosyl donor 1, 0.13 mmol of acceptor 2, 0.20 mmol of TTBP, 0.20 mmol of sulfonylating agent, THF as the solvent, 2 h of activation time. Glycosylation was run at −15 °C. Glycosylation [1] = 0.050 M.
Isolated yield.
All selectivities based on 1H NMR analysis of purified material (see SI).
Without adding TTBP.
0.20 mmol of 1, 0.1 mmol of acceptor 2, glycosylation [1] = 0.059 M. Glycosylation was run at −30 °C. TTBP = 2,4,6-tri-tert-butylpyrimidine. NR = no reaction.