Table 1.
Development of a Phosphonium Salt Alkylation Reaction
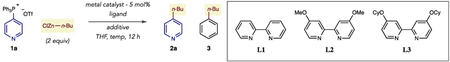 | |||||
|---|---|---|---|---|---|
| entry | catalyst system | additive | temp °C | yield 2aa | yield 3a |
| 1 | Ni(COD)2, SIPr·HCl NaOtBu | - | 50 | 37% | 20% |
| 2 | Ni(COD)2, PCy3 | - | 50 | 45% | 20% |
| 3 | Ni(COD)2, di-tBubpy | - | 50 | 37% | 22% |
| 4 | Pd(OAc)2, SIMesr·HCl NaOtBu | - | 50 | 3% | 2% |
| 5 | Pd(OAc)2, PCy3, | - | 50 | 8% | 3% |
| 6 | Co(acac)3, L1 | - | 50 | 10% | <1% |
| 7 | Co(acac)3, L1 | - | 23 | 12% | <1% |
| 8 | Co(acac)3, L2 | - | 23 | 47% | <1% |
| 9 | Co(acac)3, L2 | N-Me imidazole | 23 | 67% | <1% |
| 10 | Co(acac)3, L2 | ZnCl2 | 23 | 26% | <1% |
| 11b | Co(acac)3, L2 | N-Me imidazole | 23 | 79% | <1% |
| 12b | Co(acac)3, L3 | N-Me imidazole | 23 | 86% (78%)c | <1% |
Yields calculated by GC using 1,3,5-trimethoxybenzene as a standard. THF concentration 0.1 M.
THF concentration 0.033 M.
Isolated yield.
