Table 3.
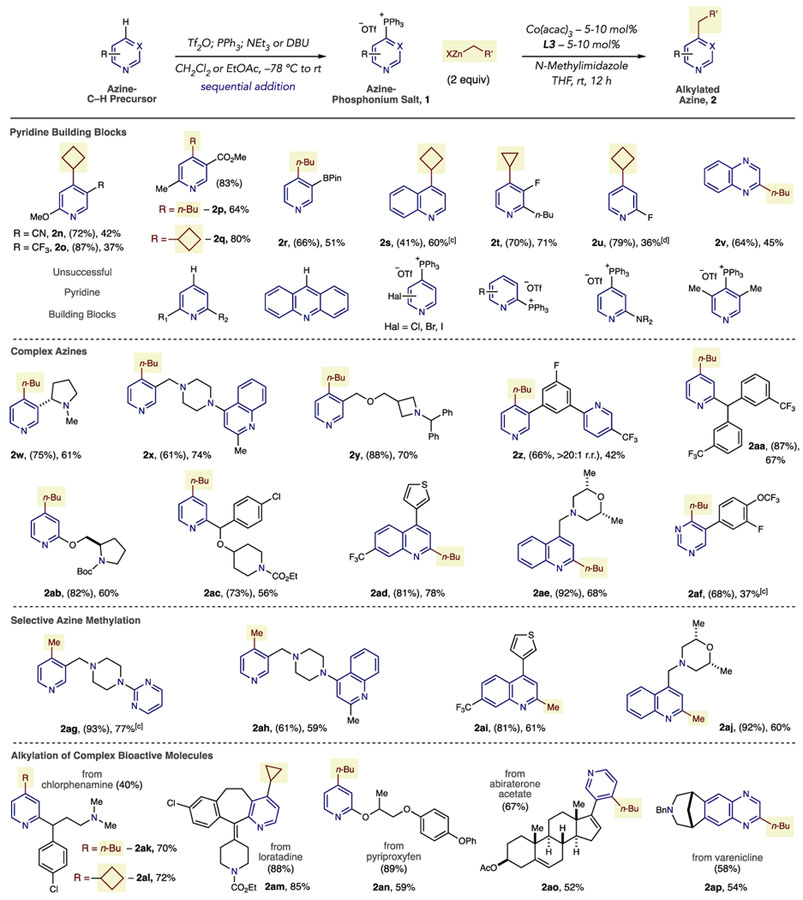
|
Typical salt-forming conditions: azine (1.0 equiv), Tf2O (1.0 equiv), PPh3 (1.1 equiv), DBU (1.1 equiv) CH2Cl2 or EtOAc, −78 °C to rt.
Isolated yields of products as single regioisomers (unless stated) are shown with yields of phosphonium salts in parentheses.
The reaction was conducted at 50 °C.
A 3.5:1 mixture of 2u and 2,4-dicyclobutylpyridine was observed in the crude 1H NMR spectrum.
