Figure 3. Suggested neuroprotective mechanisms for opioid antagonists.
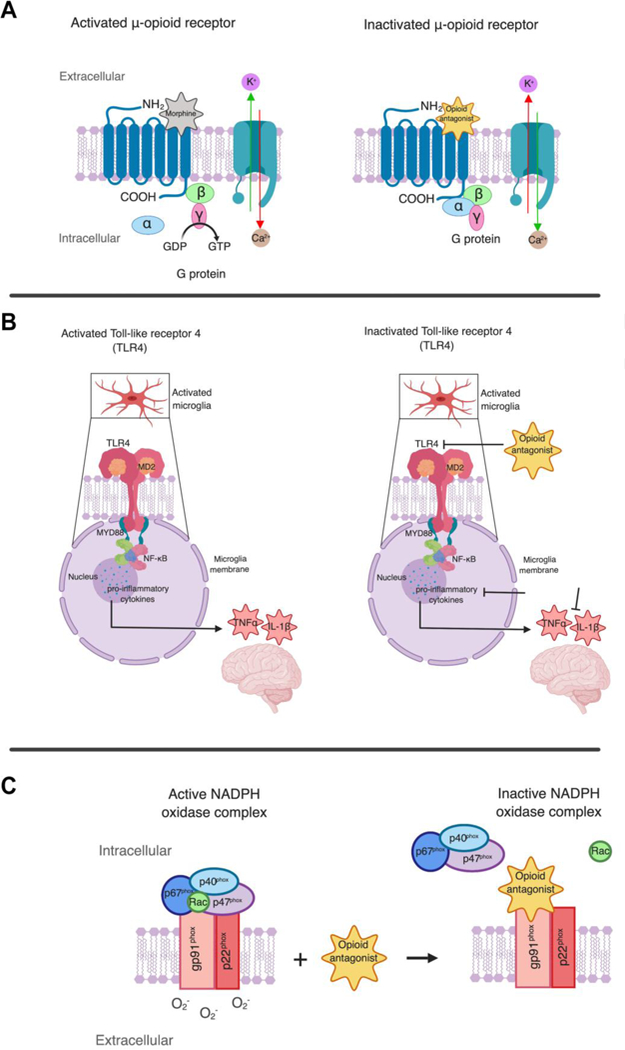
While the neuroprotective mechanisms of opioid antagonists are not clearly understood, the following mechanisms are being considered. A. μ-opioid receptors are 7 transmembrane spanning that activate G proteins composed of α, β and γ subunits which convert GDP to GTP. When activated, μ-opioid receptors exhibit inhibition of Ca2+ influx and activation of K+ channels. Opioid antagonists block μ-opioid receptor activation by competitive binding. B. TLR4 signaling pathway is activated in microgliosis. As a result, neurotoxic mediators such as TNFα and IL-1β are released. Opioid antagonists are suggested to block TLR4 signaling, leading to inhibition of pro-inflammatory cytokine production of TNFα and IL-1β. C. NADPH (dihydronicotinamide adenine dinucleotide phosphate) oxidase is an enzyme complex involved in the induction of oxidative stress that consists a membrane bound gp91phox subunit and p22phox as well as three cytosolic proteins (p40phox, p47phox, and p67phox). During an ischemic stroke, the NADPH complex is activated as and the cytosolic components are translocated to plasma membrane to interact with the membrane bound gp91phox subunit and p22phox to assemble an active NADPH oxidase enzyme complex stimulating increased superoxide O2- generation. Opioid antagonists inhibit enzymatic activity of NADPH oxidase by binding to the gp91phox subunit and induce a conformational change of the NADPH protein complex affecting the binding affinity of the cytosolic subunits p40phox, p47phox, p67phox. As a result, oxidative stress that compromises BBB integrity is reduced
