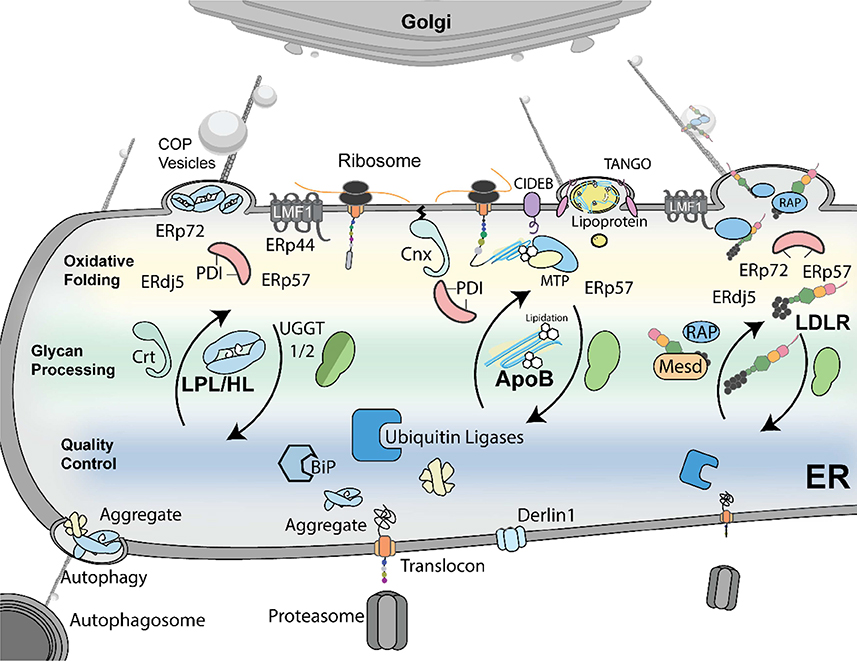
Figure 5: Quality control of LPL/HL, apoB, and LDLR in the ER.
Generalized and highly-specific chaperones are involved in oxidative folding, glycan processing, and quality control in the ER. The PDIs ERdJ5, ERp44, ERp57, ERp72, and PDI are abundant in the ER and facilitate the oxidative folding of LPL, HL, apoB, and LDLR. During oxidative folding, UGGTs glycosylate these folding proteins to promote their interactions with Crt, Cnx, and/or ERp57. In addition to these generalized chaperones, LPL/HL, apoB, and LDLR require unique chaperones to exit the ER. LMF1 is essential for the secretion of mature dimers of LPL and HL. ApoB is co-translationally lipidated and folded by MTP before leaving the ER with the help of TANGO. LDLR interacts with Mesd during folding before being chaperoned from the ER with the help of RAP. In the event of misfolding, Ubiquitin ligases and chaperones like BiP promote aggregate removal and eventual degradation by ERAD or autophagy.