Abstract
The impact of 100 μg ml−1 alumina (Al2O3) nanoparticles (NPs) on Trigonella foenum (fenugreek) in vitro cultures was studied within 3 weeks (on days 1, 7, 14, and 21) and compared with the control and bulk (macrometer-sized particles) alumina-treated groups. Transmission electron microscopy (TEM) and dynamic light scattering were used for the characterization of NPs. The results of TEM analysis represented a nearly spherical shape for the NPs with agglomeration. The zeta potential of alumina NPs was − 25.4 ± 2.5 mV and the averaged diameter was 20 ± 5 nm. Atomic absorption and inductively coupled plasma-optical emission spectroscopy provided evidence for the release and uptake of aluminum. Treatment of cultures with NPs led to an increase in the formation of lateral roots. Treatment of fenugreek with bulk alumina caused a significant decrease in the number of leaves on day 21 (p < 0.05) and the root length on days 14 and 21 compared with the control group (p < 0.05). Alumina NP has led to a significant increase in the malondialdehyde content on days 7, 14, and 21 (p < 0.001). The glutathione content was decreased significantly in NP and bulk-treated groups on days 1 and 7 (p < 0.05). FRAP assay results showed that NPs and bulk alumina led to a decrease in the antioxidant power on days 7, 14, and 21 (p < 0.001). The increased activity of catalase (p < 0.001) and ascorbate peroxidase (p < 0.001) was observed in the bulk-treated group. Lignin content had a significant increase in response to NPs on days 14 and 21 (p < 0.001). Conclusively, alumina nano/macro particles affected agronomical and physiological properties of T. foenum; however, smaller sized particles do not necessarily imply greater toxicity, while uptake of the aluminum ions should be considered seriously.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1954-7) contains supplementary material, which is available to authorized users.
Keywords: Nanoparticles, Plant in vitro culture, Fabaceae, Glutathione, Catalase, Ascorbate peroxidase
Introduction
Nanotechnology has been applied at an ever-increasing pace in various fields in recent decades. The prevalence of nanotechnology has led to the considerable exposure of nanoparticles (NPs) to our ecosystem, thereby invoking great concerns about the potential negative consequences (Tripathi et al. 2017; Zuverza-Mena et al. 2017). NPs are atomic or molecular particles with dimensions of 10–100 nm, which possess different physical and chemical features compared with the non-nano counterparts. Engineered nanoparticles (ENP) are classified into four major classes, including (1) carbon-based NPs (e.g., multi-walled carbon nanotubes), (2) metal-based NPs (e.g., quantum dots, ZnO, TiO2, Al2O3), (3) dendrimers, and (4) composites (Gottschalk et al. 2009).
Several comprehensive studies have been performed to determine which types of NPs are of priority for risk assessment (Stefaniak et al. 2013; Arts et al. 2016; Krug et al. 2018). DF4-nanogrouping framework considers all relevant features in the classification of nanomaterials, including nanomaterial’s life cycle, biological pathway, bio-persistence, uptake, and distribution as well as cellular and apical toxic effects. This classification resulted in four main groups: (1) soluble, (2) bio-persistent high aspect ratio, (3) passive, and (4) active NPs (Arts et al. 2015). Metal oxide NPs falls into the main group 1, for which risk-assessment study should be performed to evaluate the properties of NPs after dissolution in comparison to that of the bulk counterparts (Arts et al. 2016). Some metal oxides are deemed as the most hazardous NPs for our environment and prioritized for ecotoxicological studies (Krug et al. 2018). Metal oxide NPs have demonstrated distinguished physiochemical and biological characteristics, thereby being widely used for various applications (Tripathi et al. 2017). Alumina (Al2O3) NP as a metal oxide has been used abundantly in medicine, military industry, agriculture and commercial production of a number of products, such as personal care products, cosmetics, coatings, thermites, propellants, drug delivery systems, and tissue engineering (Poborilova et al. 2013; Karunakaran et al. 2016). The target tissue of aluminum damage with considerable toxicity is the human brain (Mirshafa et al. 2018). Alumina NPs are massively exposed to the terrestrial environments and can interfere with the regular growth of plants as well (Qing-Ru et al. 2006). Hence, organizations such as OECD (Organization for Economic and Cooperation Development) proposed that alumina should be considered as a nano-object for risk-assessment studies (Stefaniak et al. 2013).
The entry of NPs into the food chain is of special concern. Plants are potentially bio-accumulators of these particles; hence are more sensitive and undergo several changes when interacted with NPs, namely (a) variations in the seed germination and growth parameters (e.g., morphological aberration, or changes in biomass production) (b) alterations in the biochemical responses of plants [e.g., elevation in the activity of the oxidative stress enzymes, production of malondialdehyde (MDA), reactive oxygen species (ROS), and glycoproteins], (c) changes in the metabolite production (e.g., protein content and secondary metabolites), (d) genotoxicity, and (e) changes in the photosynthesis or quantum yield (Hatami et al. 2016; de la Rosa et al. 2017).
Trigonella foenum-graceum L. or fenugreek is a member of the Fabaceae family, being extensively used in medicine, cosmeceuticals, and foods. Several health benefits have been attributed to the fenugreek, including hypoglycemic, hypocholesterolemic, gastroprotective, and anticancer properties (Thirunavukkarasu and Anuradha 2007; Mandegary et al. 2012). In industry, it has been used for the production of steroid hormones (Basu et al. 2016; Goyal et al. 2016). This vegetable is also used as an edible flavor in Asian food regimes. Moreover, fenugreek can stint on the minimum of water and feed requirements, that’s why it adapts easily to different growth culture conditions. Fenugreek adaptability features, as well as its nutritional and medicinal values, have made it an appealing plant to cultivate all around the world. Acres of land has been dedicated to the fenugreek cultivation from Asian countries to the American ones (Acharya et al. 2006). Besides the aforementioned advantages, fenugreek has been considered as an amenable plant model for biologic research, and it is highly suited to the in vitro culture.
The current knowledge on the uptake and toxic/beneficial effects of alumina particles is limited. These studies are either performed on non-edible plants—such as Arabidopsis thaliana—or followed for a short time. It is also not clear whether the observed effects are due to the nano or bulk-sized particles; herein, fenugreek in vitro cultures during a growth period of 21 days was studied in the presence of alumina NPs and the results were compared with the bulk alumina (macro-sized particles) treated and control groups to shed light on the uptake and probable effects of alumina particles on growth parameters, variation in the stress-related responses, and the pattern of metabolite accumulation. This will result in a higher understanding of the probable hazardous/beneficial effects of nano or macro-sized alumina when these particles enter into the human food chain through an edible plant.
Materials and methods
Fenugreek seed collection and sterilization
The surface of fenugreek seeds was sterilized under a laminar airflow cabinet (Mohagheghzadeh et al. 2003); briefly soaking for 2 min in a surfactant solution, 5 min in 96% (v/v) ethanol, and 10 min in 5% (w/v) sodium hypochlorite solution, followed by 3–4 times rinsing in sterilized distilled water.
Fenugreek seed culture
Sterilized seeds were transferred onto the agar medium (0.8%). They were incubated in the culture room, at 25 ± 2 °C to promote germination (Mohagheghzadeh et al. 2009). After a week, every four seedlings were transferred to 50 ml of the hormone-free ½ Murashige and Skoog (½MS) medium to induce shoot cultures at 25 ± 2 °C under permanent light. The ½ MS medium (containing the ½ concentration of macroelements) was supplemented with 0.0002% (w/v) glycine, 0.01% (w/v) myoinositol, and 3% (w/v) sucrose. The pH of both media for seed and shoot culture was adjusted to 5.6.
Characterization of alumina particles
γ-phase nano-Al2O3 was purchased from Nanosany corporation, in which more than 99% of particles had a diameter of about 20 nm. Alumina macroparticles (bulk alumina) were purchased from Kimia Mavad Corporation (Iran) in which 70% of particles ranged from 0.06 to 0.2 mm. Morphological features of alumina NPs were analyzed through transmission electron microscopy (TEM). A suspension of 100 μg/ml of nano and bulk alumina particles was prepared in distilled water and dispersed by 1-h sonication in an ultrasonic bath (Sonorex, Bandelin, Germany). A drop of prepared dispersion was allowed to settle on formvar–carbon coated on 300 mesh copper grids for 1 min, and the excess dispersion was blotted with filter paper. The sample was dried in air and studied using a Zeiss 10C TEM (Germany) at 80 kV. The hydrodynamic size (intensity, volume, and number), polydispersity index (PDI), and zeta potential of alumina nano and macroparticles were measured by Zetasizer (ZETA-check, Microtrac, USA) at a fixed scattering angle of 90°, using a He–Ne laser (633 nm). Zeta potential was calculated based on the electrophoretic mobility in a clear disposable capillary electrophoresis cell.
Treatment of fenugreek seedlings with alumina
Seedling samples were divided into three groups. Alumina NPs were added to the ½ MS media up-to a final concentration of 100 μg/ml for the first group. Bulk alumina particles were also dispersed in the culture media of seedlings with a final concentration of 100 μg/ml as the second group. Culture media without any kind of particle were prepared as the control group. Three replicates were prepared for each sample (alumina NP, bulk alumina, and control) and all analyses were performed on days 1, 7, 14, and 21. Hence, a total of twelve samples were considered for each group to perform triplicate analyses on each day. Altogether, 36 tissue culture glass bottles containing ½ MS media were prepared initially for each set of the measurements.
Measurements of released Al3+ in the culture media and plants
The amount of released aluminum ion in the culture media, treated with nano and bulk alumina particles, were measured by GBC 932 AA (Australia) flame atomic absorption spectrometer, equipped with a hollow cathode lamp and a deuterium background collector at 396.2 nm using nitrous oxide flame. The suspension was ultrasonicated and the pH was adjusted on 2.0 prior to atomic absorption spectroscopy (AAS). Samples of plants were oven-dried to quantify the Al concentration in nanoparticle treated groups (n = 3) and bulk alumina-treated groups (n = 3). Microwave acid digestion was performed by the addition of a 4:1 (v/v) ratio of HNO3:H2O2 to 500 mg of dried samples in closed-microwave vessels. Vessels were remained at 25 °C for 10 min after shaking. Digestion parameters for the microwave oven (Milestone Ethos Up, SK-15 high pressure rotor) included heating at 200 °C for 15 min at a total power of 1800 W, followed by cooling. Digested samples were dissolved in 50 ml of Millipore water and were analyzed for the presence of aluminum by inductively coupled plasma-optical emission spectroscopy (ICP-OES, Spectro Arcos, SPECTRO, Germany) according to Fathabad et al. (2018).
Measurement of growth parameters
Growth parameters were measured for the replicates of each treatment. The length of the grown stem and roots were measured in centimeters. Changes in fresh weight (FW) and dry weight (DW) of plantlets were measured for triplicates of each group on days 1, 7, 14, and 21. To calculate dry biomass, samples were dried in an oven at 40 °C for at least 48 h.
Malondialdehyde (MDA) content measurement (TBARS assay)
The MDA content was measured through a reaction with thiobarbituric acid (TBA) (Heath and Packer 1968). Five hundred mg of plant tissue was homogenized in cold ethanol (80% v/v). Homogenized samples were centrifuged at 11,200×g for 15 min. The supernatant was mixed with 0.5% (w/v) of TBA in 20% (w/v) trichloroacetic acid (TCA) and the mixture was placed in a water bath at 90 °C for 1 h. Then samples were cooled in an ice bath to stop the reaction and centrifuged at 11,200×g for 5 min. The absorbance of the mixture was measured at 532 and 600 nm by spectrophotometer (Ultraspec 2000 UV Pharmacia, Biotech, Sweden). MDA concentration was measured according to the absorbance of the MDA–TBA complex. This absorbance was estimated by subtracting the non-specific absorption at 600 nm from the absorption at 532 nm. The results of the TBARS assay were normalized according to the concentration of total protein and reported as the nmol of MDA/mg protein. The measurements were performed in triplicates for each group on days 1, 7, 14, and 21.
Glutathione (GSH) content measurement
Five hundred mg of triplicate samples related to the nano and bulk-treated groups and the control group on days 1, 7, 14, and 21, were homogenized in 1.15% (w/v) KCl using a homogenizer. The GSH content was measured through an oxidation–reduction reaction with DTNB (Smith et al. 1988). Briefly, 5 ml of homogenized sample was mixed with 1 ml of 50% (w/v) TCA and 4 ml of distilled water. The mixture was vigorously shaken and centrifuged at 11,200×g for 20 min at 4 °C. Two ml of the supernatant was added to Tris buffer (0.1 M pH = 7.5) followed by adding 100 μl of 0.01 M DTNB to the mixture. All steps were performed under cold conditions. The absorbance of the yellowish mixture was measured at 412 nm.
Measurement of antioxidant power using the FRAP assay
The antioxidant power was assessed based on the ferric ion reducing antioxidant power (FRAP) assay (Benzie and Strain 1996). The FRAP agent included 2.5 ml of 300 mM acetate buffer (pH = 3), 0.25 ml of 20 mM FeCl3, and 0.25 ml of 10 mM TPTZ (2,4,6-Tri(2-pyridyl)-s-triazine) in 40 mM HCl. A hundred μl of samples resulted from the homogenization of 500 mg plant tissue in KCl was added to the FRAP agent and incubated at room temperature for 4 min. Then, the mixture was centrifuged at 11,200×g for 1 min and the absorbance was measured at 593 nm. The measurements were performed in triplicates for each group on days 1, 7, 14, and 21.
Measurement of catalase (CAT) and ascorbate peroxidase (APX) enzyme activity
Fresh plantlets were rinsed with 5% (w/v) CaCl2 and distilled water. Five hundred mg of triplicate samples related to the nano and bulk-treated groups and the control group on days 1, 7, 14, and 21 were homogenized in 25 mM KH2PO4 in a ratio of 25% (w/v) followed by centrifuging at 10,000×g, 4 °C for 8 min. The supernatant was used as an extract for further assays.
To determine the CAT activity (CAT, EC 1.11.1.6), decomposition of H2O2 at 240 nm was assessed (Shangari and O’Brien 2006). The reaction occurred for 3 min in a mixture containing 950 μl of 10 mM H2O2 and 50 μl of plant extract.
To determine APX activity, 100 μl of plant extract was added to a mixture containing 886 μl of 0.1 M KH2PO4, 4 μl of 25 mM ascorbate, and 10 μl of 17 mM H2O2. The absorbance of the reaction mixture was measured at 265 nm (Amako et al. 1994).
Lignin and protein content measurement
To retrieve cell wall, triplicate samples treated with nano or macroparticles from days 1, 7, 14, and 21 were homogenized separately in 80% (v/v) ethanol using a homogenizer (Heidolph, Merck, Germany). Homogenized samples were rinsed with 80% (v/v) ethanol in a water bath at 80 °C for 20 min, followed by centrifugation at 2000×g for 30 min and discarding the supernatant. These steps were performed five times for each sample until they became clear. Then, 1 ml acetone was added to each sample, the supernatant was discarded and samples were dried in the oven.
Lignin content was measured according to the Klason method (Browning 1967). A 150 mg extracted cell wall was mixed with 4.5 ml of 72% (v/v) H2SO4 and incubated in a water bath at 30 °C for 30 min. Distilled water (76.5 ml) was added to each sample to dilute H2SO4 from 72% (v/v) to 4% (v/v). Samples were autoclaved at 120 °C for 1 h and filtered through a Buchner funnel using a vacuum pump. Precipitates on filter paper were dried and weighted to measure the lignin content. Total protein content was measured according to the Bradford method (Bradford 1976).
Metabolite pattern of plants
UV spectroscopy of the methanolic extract of dried plants was used to determine changes in the pattern of metabolites. To prepare the methanolic extract, 500 μl of 50% (v/v) methanol was added to 100 mg of the plant. Plants were squeezed manually and incubated at 65 °C for 1 h in a heating block (DAIHAN scientific, South Korea). Samples were then centrifuged at 10,000×g for 15 min. The absorbance of the supernatant was measured spectrophotometrically in the range of 200–700 nm.
Statistical analysis
An analysis of variance (ANOVA) was performed using the Tukey multiple comparison test at a significant level of 0.05 and 0.001. The relevant graphs were drawn with the Graph Pad Prism software.
Results
Characterization of alumina particles
Morphological features, particle size distribution, and the surface charge of alumina particles were characterized. The results of TEM analysis represented a nearly spherical shape for the NPs (Fig. 1). Moreover, a mere agglomeration could be observed in the TEM results. The zeta potential of alumina NPs was − 25.4 ± 2.5 mV, denoting an incipient instability behavior for the colloid. The number averaged diameter was 20 ± 5 nm, in agreement with the size that was stated by the supplier. The PDI was 0.19. Measurement of bulk alumina particles confirmed a size greater than one micron. The zeta potential of bulk alumina was − 60.5 ± 3.5 mV, indicating higher instability compared with the alumina NP-treated group.
Fig. 1.

Transmission electron microscopy image of alumina nanoparticle dispersion in distilled water at 100 nm
Measurement of released Al3+ in the culture media and plants
To confirm the release of aluminum upon dissolving alumina particles in the culture media, the aluminum concentration was measured by flame AAS. According to the results, the amount of aluminum ion released from dispersing alumina NPs in the culture media far surpassed that one from the dispersion of alumina bulk particles. The amount of aluminum ion in the media containing bulk alumina-treated group was a mere amount of 0.25 ppm, whereas this amount in a media with alumina NP-treated group was a massive amount of 5 ppm. To determine the plant’s uptake of aluminum, ICP-OES was performed on microwave digested samples. The amount of aluminum uptake increased proportionally as the plants grow from day 7 to 21 in both nano alumina- and bulk alumina- treated groups. On day 7, the uptake amount in the bulk alumina- treated group was a mere amount of 4.8 μg/g unlike the NP-treated group, which had an uptake of 11.4 μg/g. Nevertheless, on days 14 and 21, the trend was reversed. The amount of aluminum in NP-treated groups was 19.4 μg/g and 42.4 μg/g on days 14 and 21, respectively, while in the bulk alumina-treated group was 28.4 μg/g and 85 μg/g.
Effects of alumina on growth parameters of fenugreek in vitro cultures
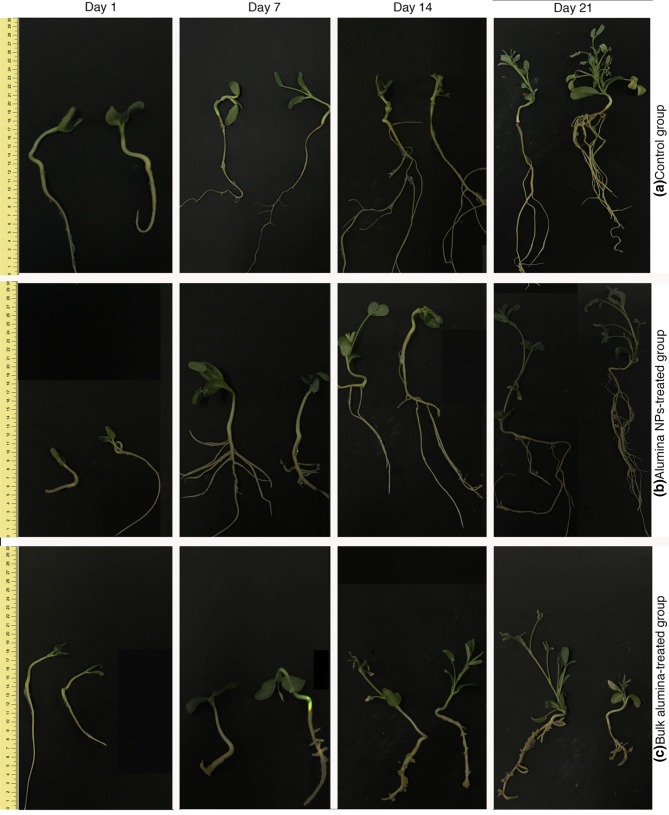
The effect of alumina particles on the growth parameters of fenugreek in vitro cultures was assessed within 3 weeks on days 1, 7, 14, and 21. Changes in morphological features, including stem growth, root elongation, and formation of lateral roots is shown in Figs. 2 and 3. On the first day, the alumina NP-treated group did not show any significant change in growth parameters compared with the control and bulk alumina-treated groups. Surprisingly, on days 7, 14, and 21, the bulk alumina-treated group had a decline in growth, and the formation of lateral roots could be hardly observed in this group.
Fig. 2.
Effect of alumina nanoparticles (NPs) on the morphology of fenugreek in vitro cultures on days 1, 7, 14, and 21. a Control group, b alumina NP-treated group, c bulk alumina-treated group. One replicate is shown out of triplicate samples
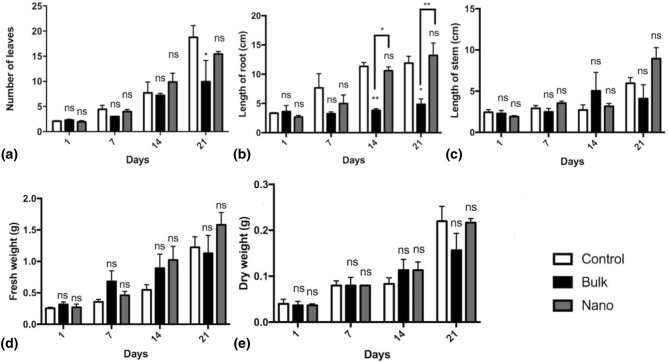
Fig. 3.
Effect of alumina nanoparticles (NPs) on a the number of leaves, b root length, c stem length, d fresh weight, e dry weight of fenugreek in vitro cultures in comparison with the bulk alumina-treated and control groups. Data are represented as mean ± SEM within 21 days. “Asterisks” and “ns” represent a significant and non-significant difference of mean values of three replicates in comparison with the control, respectively (p ≤ 0.05). The higher number of asterisks represents a lower p value
Statistical analysis of growth parameters revealed that the treatment of fenugreek with alumina NPs did not lead to a significant increase in the number of leaves or the length of roots compared with the control group (Fig. 3a, b). In contrast, treatment of fenugreek with bulk alumina caused a statistically significant decrease in the number of leaves on day 21 (p < 0.05) and the root length on days 14 and 21 compared with the control group (p < 0.05). Moreover, the difference between the root length of the alumina NP-treated group and bulk alumina-treated group on days 14 and 21 was statistically noticeable (p < 0.05) (Fig. 3b). An increase in the stem length on days 7, 14, and 21 was observed in the alumina NP-treated group compared with the control group. However, this increase was not statistically significant (Fig. 3c). Similar results were observed in the assessment of biomass. In effect, treatment of fenugreek with alumina NPs led to elevated FW on days 14 and 21 in comparison with the control group; nevertheless, the mentioned rise was not statistically significant (Fig. 3d, e).
Effect of alumina NPs on lipid peroxidation and GSH content in fenugreek in vitro cultures
The measurement of MDA content revealed that treatment of fenugreek with alumina NPs has led to a significant increase in the MDA content on days 7, 14, and 21 (p < 0.001) compared with the control and bulk alumina-treated groups (Fig. 4a), denoting the occurrence of a significant lipid peroxidation on these days. The redox GSH was decreased significantly in alumina NP- and bulk alumina-treated groups on days 1 and 7 compared with the control group (p < 0.05); however, the reduction on days 14 and 21 was not statistically significant (Fig. 4b).
Fig. 4.
Effect of alumina nanoparticles (NPs) on a lipid peroxidation, b GSH content of fenugreek in vitro cultures in comparison with the bulk alumina-treated and control groups. Data are represented as mean ± SEM within 21 days. “Asterisks” and “ns” represent a significant and non-significant difference of mean values of three replicates in comparison with the control, respectively (p < 0.001 for part a and p ≤ 0.05 for part b)
Evaluation of antioxidant capacity and antioxidant enzyme’s activity of fenugreek in vitro cultures
The CAT activity in the alumina NP- and bulk alumina-treated groups increased during the whole assessment. However, a statistically significant increase in the CAT activity was observed in the bulk alumina-treated group in comparison with the two other groups on all days (p < 0.001) (Fig. 5a). The measurement of APX activity demonstrated a significant increase in the activity of this enzyme in the bulk alumina-treated group only on day 7 (p < 0.001) (Fig. 5b). Although the activity of APX in the alumina NP-treated group on the seventh day was higher than the control group, the difference was not statistically significant. FRAP assay results showed that the treatment of fenugreek with either alumina NPs or bulk alumina led to a decrease in its antioxidant power. This decrease was statistically significant in the aforementioned groups in comparison with the control group on days 7, 14, and 21 (p < 0.001). Moreover, on day 21, a significant difference in the antioxidant power reduction between the alumina NP-treated group and bulk alumina-treated group was observed (p < 0.05) (Fig. 5c).
Fig. 5.
Effect of alumina nanoparticles (NPs) on a catalase activity, b ascorbate peroxidase activity, c antioxidant power of fenugreek in vitro cultures in comparison with the bulk alumina-treated and control groups. Data are represented as mean ± SEM within 21 days. “Asterisks” and “ns” represent a significant and non-significant difference of mean values of three replicates in comparison with control, respectively. The p value in all comparisons is less than 0.001 except the p value for comparison of the bulk alumina-treated group and alumina NP-treated group in the FRAP assay, which is less than 0.05 (shown by a)
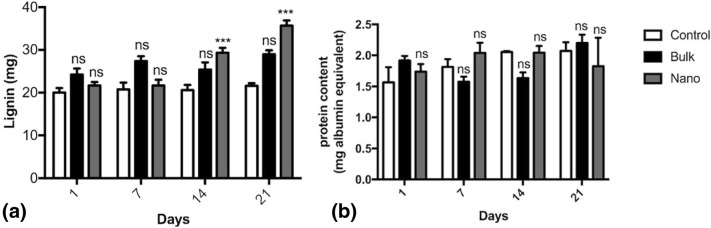
Effect of alumina NPs on lignin and protein content of fenugreek in vitro cultures
The lignin and protein content of fenugreek in vitro cultures on days 1, 7, 14, and 21 of growth was measured according to the standard methods of Klason and Bradford, respectively. Measurement of Klason lignin revealed that bulk alumina elevated lignin content during the whole treatment; however, NPs resulted in a significant increase in the lignin content on days 14 and 21 in comparison with the control group (p < 0.001) (Fig. 6a). It was disclosed that there were no significant changes in the protein content of alumina NP-treated groups in comparison with the control or bulk alumina-treated group during characterization (Fig. 6b).
Fig. 6.
Effect of alumina nanoparticles (NPs) on a lignin content, b protein content of fenugreek in vitro cultures in comparison with the bulk alumina-treated and control groups. Data are represented as mean ± SEM within 21 days. “Asterisks” and “ns” represent a significant and non-significant difference of mean values of three replicates in comparison with the control, respectively (p ≤ 0.001)
UV absorbance pattern of fenugreek in vitro cultures
To verify the probable changes in the pattern of metabolites after treatment, the UV absorbance of the methanolic extracts derived from fenugreek in vitro cultures was measured in the presence of alumina NPs and bulk alumina within 21 days. Results were compared to the relevant data of the control group. It was shown that the maximum absorbance, as well as the absorbance pattern of either the alumina NP-treated group or the bulk alumina-treated group on all days of the experiment, was similar to that of the control group. However, two maximum absorptions at 220–250 nm and 320–330 nm were notable in all groups (Online Resource 1).
Discussion
High demand for alumina NPs and their importance in risk-assessment studies
The estimated exposure of alumina NPs to the environment is determined as high to medium (Krug et al. 2018). This fact prompt us to perform risk-assessment studies to elucidate the hazardous effects of these particles on an edible crop like fenugreek. Several factors, including size, contact angle, zeta potential, concentration, and surface characteristics determine the toxicity of alumina NPs (Asztemborska et al. 2015; Karunakaran et al. 2015; Mui et al. 2016). γ-alumina NPs, ranging from 5 to 30 nm, are soluble in the pH range of 3–12 and form a stable colloid under a pH of 7.5–8 (Mui et al. 2016). Therefore, the merely observed aggregation and colloidal instability in our study can be attributed to the selected pH of colloid, which was 5.6. Although a stable suspension of alumina particles either in the NP or bulk-treated group was not formed, AAS confirmed the release of aluminum in the media containing either nano- or macroparticles. According to the ICP-OES results, aluminum uptake could be detected on days 7, 14, and 21 in the plants treated with nano and bulk particles which showed an increase in a time-dependent manner. However, the amount of penetrated aluminum on days 14 and 21 in the bulk alumina-treated group exceeded the NP-treated group. Nevertheless, both AAS and ICP-OES prove the release, exposure, and uptake of aluminum by fenugreek. Although alumina NPs of 20 nm size, deemed to have a distinguished sorption capability in contrast to their bulk counterpart, TEM analysis represented a mere agglomeration of alumina NPs, which might decrease the bioavailability of particles, concurring with the ICP-OES results (Fig. 1). Moreover, aluminum can bind to different salts in the media (Bhoomika et al. 2013).
Effects of alumina particles on growth parameters
Stress-induced morphogenic response (SIMR) can be triggered under a toxic amount of aluminum derivatives. Root elongation has been considered as one of the standard indicators of phytotoxicity by the U.S. Environmental Protection Agency (EPA) (Tripathi et al. 2017). Herein, various growth parameters related to the phytotoxicity of alumina have been studied, among which changes in the pattern of root elongation is the most prevalent physiological response (Santos et al. 2014). As mentioned in the results, bulk alumina resulted in a decline in growth and lateral root formation significantly, which is in line with the higher penetrated amount of bulk aluminum observed in our study especially on days 14 and 21. The decline in the growth of roots, inhibition of cell elongation and cell division in the plant roots and lower productivity under aluminum treatment is also documented in other studies. Aluminum down-regulates the biosynthesis of auxin which could have a role in the inhibition of root growth in the bulk alumina-treated group (Kumari et al. 2008). Although alumina NPs at a concentration of 100 μg/ml yielded an increase in the root length of fenugreek on days 14 and 21 (Figs. 2, 3), this observation was not statistically significant. Results highly depend on the concentration and size of the studied particles (Juhel et al. 2011; Yanık and Vardar 2015). In our study, alumina NPs might have enhanced the gas transfer leading to an increase in the growth of fenugreek in comparison with the control group, although it was not significant. We supposed that a fraction of aluminum is bound to the salts in the media; hence the bioavailability of aluminum, as well as the nutrients in the media was changed leading to the difference in the growth of fenugreek. The formation of lateral root on day 14 of treatment in the alumina NP-treated groups advocates the previous contention. Although alumina NPs augmented root elongation in fenugreek, bulk alumina at the same concentration posed a negative effect on growth. As mentioned earlier, as the size of particles decreases, their characteristics differ (Jin et al. 2017). Moreover, the toxicity of alumina NPs in comparison with their micro- or macro- counterparts might be revealed at higher concentrations (Karunakaran et al. 2015).
Oxidative stress-related responses to alumina NPs
Results from our study along with others demonstrate that the oxidative stress responses are incrementally generated as the dose and exposure duration of alumina particles increase. Oxidative stress responses are usually measured through several approaches, including the measurement of MDA (TBARS assay) and GSH contents as well as the activity and power of antioxidant enzymes. CAT and APX are two key enzymes in coping with oxidative stress. CAT eliminates the majority of produced ROS; however, APX is able to defeat ROS not removed by CAT even at the minimum amounts (Ghanati et al. 2005). An indicator of oxidative-related response is lipid peroxidation, which can be revealed by the measurement of the MDA content. We have shown that 100 μg/ml alumina NPs, which was far less than the previously studied concentrations, cause lipid peroxidation and an increase in the CAT activity in fenugreek in vitro cultures from the initial days of treatment. In effect, the MDA content in alumina NP-treated groups significantly increased on days 7, 14, and 21 (Fig. 4a). CAT, as the fundamental enzyme in defeating ROS, was activated substantially in response to both nano and bulk particles with a significant increase in the bulk-treated group (Fig. 5a). Nonetheless, APX, as the spare enzyme in coping with ROS, was activated consequentially on day 7 in the alumina bulk-treated group (Fig. 5b). Previous studies have shown that alumina NPs cause lipid peroxidation and an increase in the CAT activity in a dose-dependent manner in N. tabacum (Yanık and Vardar 2018).
One might propound the question that the exerted oxidative response is attributed to the alumina at nano-size or related to the Al3+ ions. It has been claimed that the released Al3+ plays a significant role in cytotoxicity (Poborilova et al. 2013). In our study, the CAT activity increased both in the alumina NP- and bulk alumina-treated groups—with a significant increase in the bulk-treated group—which is in agreement with the higher penetration of the aluminum in the bulk-treated group corroborating the idea of aluminum ion as the main cause of oxidative stress. The significant elevation of CAT and APX activity in the bulk-treated group is in line with the clues arguing the up-regulation of genes/enzymes in species that are sensitive to aluminum and is encountered as a sign of Al toxicity (Liu et al. 2008). However, when anti-peroxidation enzymes are triggered the oxidative injury of aluminum could be refined to some extent. In other words, this contention relegates the effect of nano-size alumina.
GSH is another important defensive compound against oxidative stress. As the oxidative stress increases, the GSH resources of the plant deplete and it becomes more vulnerable to the stress. In this study, the GSH content significantly decreased in the alumina NP- and bulk alumina-treated groups immediately after exposure. This observation suggests the GSH-dependent system as the first oxidative–reductive system coping with alumina stress. Besides GSH depletion in fenugreek cultures, the FRAP analysis demonstrated a decrease in the antioxidant power from day 7 of the treatment in all treated groups.
Other effects caused by alumina nano- and macroparticles
Few studies have been carried out to illuminate the potential effects of nano-sized particles on metabolite biosynthesis. In our study, the total protein content was not changed in response to the treatment with alumina NPs. The comparative proteomic analysis demonstrated a rise in various proteins in response to Al exposure in A. thaliana (Mustafa and Komatsu 2016), B. oleraceae (Amist et al. 2017), and S. lycopersicon (Ahmed et al. 2018). Alumina NPs led to a decline of protein content in T. aestivum at low concentration; however, the protein content increased upon an upswing in the concentration of alumina NPs (Yanık and Vardar 2015). In our study, it can be proposed that an increase in the stress-related enzymes was offset by a decline in other proteins as a defensive mechanism in the plant; thereby, the total protein content remained constant.
The effects of alumina NPs on the pattern of primary and secondary metabolites also have been less subject of study. The lignin content increased in the bulk alumina-treated group; however, this elevation was significant on days 14 and 21 in fenugreek cultures in response to alumina NPs. This observation can be attributed to the increased oxidative stress in the cells, wherein oxidation and radical formation of monomers responsible for the lignin polymerization occurs. It is demonstrated that in the presence of laccases/peroxidases, radicals are formed from coumaryl, coniferyl, and sinapyl alcohols (Hemmati et al. 2007; Petersen et al. 2018). Then, radical–radical coupling results in the growth of lignin polymer. The increased amount of lignin biosynthesis has been reported under aluminum stress as a result of the up-regulation of genes involved in the biosynthetic pathway of phenylpropanoids or transcription factors of the corresponding pathway (Zhou et al. 2016). UV analyses showed similar patterns of metabolites in all groups at each point. Observation of maximum absorption at about 220–250 nm and 320–330 nm could be attributed to various metabolites, such as trigocoumarins (Parmar et al. 1982), which has been reported in the aerial parts of T. foenum. It has been demonstrated that aluminum ions are able to form complexes with secondary metabolites in planta. In other words, these compounds, in turn, might be involved in the internal detoxification of aluminum (Ezaki et al. 2013). However, advanced spectroscopic and chromatographic analyses are proposed for the in-depth elucidation of the identity and probable changes of the metabolites.
Conclusion
This study aimed to compare the physiological and biochemical effects of nano and macro-sized alumina on the edible plant T. foenum. Our results revealed that alumina NPs at low concentrations do not impose a negative effect on growth parameters. Nevertheless, the bulk alumina particles deterred growth at some stages in the experiment. It can be contended that alumina features differ thoroughly as the size of the particle decreases. Although substantial lipid peroxidation occurred in the alumina NP-treated groups all the oxidative stress-related pathways were activated in response to the alumina macroparticles. The greater importance of CAT in defeating ROS was emphasized in our observation. Results obtained by this study along with others provided a clear idea of the allowed amount of alumina NPs that can be exposed to the terrestrial environment without causing any detrimental effects. Our results proved that alumina macroparticles could induce higher toxicity symptoms than the nano counterpart. Nonetheless, there is a lack of knowledge explaining the effects of alumina NPs on the production of important nutritional and medicinal metabolites. Hence, it is suggested to implement further investigation to evaluate these effects on plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Effect of alumina NPs on the UV absorbance of methanolic extract derived from fenugreek in vitro cultures within 21 days, in comparison with the bulk alumina-treated and control groups. (a) day 1, (b) day 7, (c) day 14, and (d) day 21. (TIFF 11738 kb)
Acknowledgements
Authors would like to thank Shiraz University of Medical Sciences, Shiraz, IRAN for Grant Nos. 95-01-05-12926 and 1396-01-05-14613. This work was part of the Pharm D thesis of M. Hakimzadeh and D. Mansouri. Authors would like to thank Dr. S. Abolmaali and Dr. S. Nazmara for DLS and ICP-OES analyses, respectively.
Abbreviations
- NP
Nanoparticles
- TEM
Transmission electron microscopy
- DLS
Dynamic light scattering
- MDA
Malondialdehyde
- TCA
Trichloroacetic acid
- TBA
Thiobarbituric acid
- FW
Fresh weight
- DW
Dry weight
- FRAP
Ferric ion reducing antioxidant power
- ROS
Reactive oxygen species
- PDI
Polydispersity index
- ENP
Engineered nanoparticles
- CAT
Catalase
- APX
Ascorbate peroxidase
- GSH
Glutathione
- AAS
Atomic absorption spectroscopy
- ICP-OES
Inductive coupled plasma-optical emission spectroscopy
Author contributions
SH has conceived and designed the experiments. RH and MH performed the experiments. HO and SH prepared the manuscript. SH has revised the manuscript. All authors proofread the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Acharya S, Thomas J, Basu S. Fenugreek: an “old world” crop for the “new world”. Biodiversity. 2006;7(3–4):27–30. doi: 10.1080/14888386.2006.9712808. [DOI] [Google Scholar]
- Ahmed B, Khan MS, Musarrat J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): a study on growth dynamics and plant cell death. Environ Pollut. 2018;240:802–816. doi: 10.1016/j.envpol.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Amako K, Chen GX, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35(3):497–504. doi: 10.1093/oxfordjournals.pcp.a078621. [DOI] [Google Scholar]
- Amist N, Singh NB, Yadav K, Singh SC, Pandey JK. Comparative studies of Al3+ ions and Al2O3 nanoparticles on growth and metabolism of cabbage seedlings. J Biotechnol. 2017;254:1–8. doi: 10.1016/j.jbiotec.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Arts JH, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) Regul Toxicol Pharmacol. 2015;71(2):S1–S27. doi: 10.1016/j.yrtph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Arts JH, Irfan MA, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Neubauer N, Petry T, Sauer UG. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul Toxicol Pharmacol. 2016;76:234–261. doi: 10.1016/j.yrtph.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Asztemborska M, Steborowski R, Kowalska J, Bystrzejewska-Piotrowska G. Accumulation of aluminium by plants exposed to nano- and microsized particles of Al2O3. Int J Environ Res. 2015;9(1):109–116. doi: 10.22059/ijer.2015.880. [DOI] [Google Scholar]
- Basu SK, Cetzal-IX W, Zandi P (2016) Forage fenugreek (Trigonella foenum-graecum L.) production: a boon for semi-arid agricultural regions. In: VII international scientific agriculture symposium,” Agrosym 2016”, 6–9 Oct 2016, Jahorina, Bosnia and Herzegovina. Proceedings University of East Sarajevo, Faculty of Agriculture
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhoomika K, Pyngrope S, Dubey R. Differential responses of antioxidant enzymes to aluminum toxicity in two rice (Oryza sativa L.) cultivars with marked presence and elevated activity of Fe SOD and enhanced activities of Mn SOD and catalase in aluminum tolerant cultivar. Plant Growth Regul. 2013;71(3):235–252. doi: 10.1007/s10725-013-9824-5. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning BL (1967) Methods of wood chemistry. vol. II. Interscience Publishers, a Division of John Wiley and Sons Inc, New York
- de la Rosa G, García-Castañeda C, Vázquez-Núñez E, Alonso-Castro ÁJ, Basurto-Islas G, Mendoza Á, Cruz-Jiménez G, Molina C. Physiological and biochemical response of plants to engineered NMs: implications on future design. Plant Physiol Biochem. 2017;110:226–235. doi: 10.1016/j.plaphy.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Ezaki B, Jayaram K, Higashi A, Takahashi K. A combination of five mechanisms confers a high tolerance for aluminum to a wild species of Poaceae, Andropogon virginicus L. Environ Exp Bot. 2013;93:35–44. doi: 10.1016/j.envexpbot.2013.05.002. [DOI] [Google Scholar]
- Fathabad AE, Shariatifar N, Moazzen M, Nazmara S, Fakhri Y, Alimohammadi M, Azari A, Khaneghah AM. Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: a risk assessment study. Food Chem Toxicol. 2018;115:436–446. doi: 10.1016/j.fct.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Ghanati F, Morita A, Yokota H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil. 2005;276(1–2):133–141. doi: 10.1007/s11104-005-3697-y. [DOI] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 2009;43(24):9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Goyal S, Gupta N, Chatterjee S. Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J Toxicol. 2016 doi: 10.1155/2016/1250387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami M, Kariman K, Ghorbanpour M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci Total Environ. 2016;571:275–291. doi: 10.1016/j.scitotenv.2016.07.184. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hemmati S, Schneider B, Schmidt TJ, Federolf K, Alfermann AW, Fuss E. Justicidin B 7-hydroxylase, a cytochrome P450 monooxygenase from cell cultures of Linum perenne Himmelszelt involved in the biosynthesis of diphyllin. Phytochemistry. 2007;68(22–24):2736–2743. doi: 10.1016/j.phytochem.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fan X, Li X, Zhang Z, Sun L, Fu Z, Lavoie M, Pan X, Qian H. Distinct physiological and molecular responses in Arabidopsis thaliana exposed to aluminum oxide nanoparticles and ionic aluminum. Environ Pollut. 2017;228:517–527. doi: 10.1016/j.envpol.2017.04.073. [DOI] [PubMed] [Google Scholar]
- Juhel G, Batisse E, Hugues Q, Daly D, van Pelt FN, O’Halloran J, Jansen MA. Alumina nanoparticles enhance growth of Lemna minor. Aquat Toxicol. 2011;105(3):328–336. doi: 10.1016/j.aquatox.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Karunakaran G, Suriyaprabha R, Rajendran V, Kannan N. Toxicity evaluation based on particle size, contact angle and zeta potential of SiO2 and Al2O3 on the growth of green algae. Adv Nano Res. 2015;3(4):243–255. doi: 10.12989/anr.2015.3.4.243. [DOI] [Google Scholar]
- Karunakaran G, Suriyaprabha R, Rajendran V, Kannan N. Influence of ZrO2, SiO2, Al2O3 and TiO2 nanoparticles on maize seed germination under different growth conditions. IET Nanobiotechnol. 2016;10(4):171–177. doi: 10.1049/iet-nbt.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug HF, Bohmer N, Kühnel D, Marquardt C, Nau K, Steinbach C. The DaNa2.0 knowledge base nanomaterials—an important measure accompanying nanomaterials development. Nanomaterials. 2018;8(4):204. doi: 10.3390/nano8040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Taylor GJ, Deyholos MK. Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol Genet Genomics. 2008;279(4):339. doi: 10.1007/s00438-007-0316-z. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yang J, He L, Li Y, Zheng S. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plant. 2008;52(1):87–92. doi: 10.1007/s10535-008-0014-7. [DOI] [Google Scholar]
- Mandegary A, Pournamdari M, Sharififar F, Pournourmohammadi S, Fardiar R, Shooli S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem Toxicol. 2012;50(7):2503–2507. doi: 10.1016/j.fct.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Mirshafa A, Nazari M, Jahani D, Shaki F. Size-dependent neurotoxicity of aluminum oxide particles: a comparison between nano-and micrometer size on the basis of mitochondrial oxidative damage. Biol Trace Elem Res. 2018;183(2):261–269. doi: 10.1007/s12011-017-1142-8. [DOI] [PubMed] [Google Scholar]
- Mohagheghzadeh A, Hemmati S, Mehregan I, Alfermann AW. Linum persicum: lignans and placement in Linaceae. Phytochem Rev. 2003;2(3):363–369. doi: 10.1023/B:PHYT.0000045501.97438.9c. [DOI] [Google Scholar]
- Mohagheghzadeh A, Dehshahri S, Hemmati S. Accumulation of lignans by in vitro cultures of three Linum species. Z Naturforsch C. 2009;64(1–2):73–76. doi: 10.1515/znc-2009-1-213. [DOI] [PubMed] [Google Scholar]
- Mui J, Ngo J, Kim B. Aggregation and colloidal stability of commercially available Al2O3 nanoparticles in aqueous environments. Nanomaterials. 2016;6(5):90. doi: 10.3390/nano6050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa G, Komatsu S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim Biophys Acta. 2016;1864(8):932–944. doi: 10.1016/j.bbapap.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Parmar VS, Jha HN, Sanduja SK, Sanduja R. Trigocoumarin—a new coumarin from Trigonella foenum graecum. Z Naturforschung B. 1982;37(4):521–523. doi: 10.1515/znb-1982-0423. [DOI] [Google Scholar]
- Petersen M, Hans J, Matern U. Biosynthesis of phenylpropanoids and related compounds. Annual Plant Rev. 2018;40:182–257. doi: 10.1002/9781119312994.apr0426. [DOI] [Google Scholar]
- Poborilova Z, Opatrilova R, Babula P. Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ Exp Bot. 2013;91:1–11. doi: 10.1016/j.envexpbot.2013.03.002. [DOI] [Google Scholar]
- Qing-Ru Z, Bo-Han L, Li-Tian Z, Xi-Hong Z, Hong-Xiao T. Short-term alleviation of aluminum phytotoxicity by urea application in acid soils from south China. Chemosphere. 2006;63(5):860–868. doi: 10.1016/j.chemosphere.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Santos C, Silva S, Pinto-Carnide O. Chapter six—Aluminum phytotoxicity: physiological approaches and tolerance. In: Fishbein JC, Heilman JM, editors. Advances in molecular toxicology. Amsterdam: Elsevier; 2014. pp. 203–236. [Google Scholar]
- Shangari N, O’Brien PJ. Catalase activity assays. Curr Protoc Toxicol. 2006;27:1–16. doi: 10.1002/0471140856.tx0707s27. [DOI] [PubMed] [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175(2):408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Hackley VA, Roebben G, Ehara K, Hankin S, Postek MT, Lynch I, Fu WE, Linsinger TP, Thünemann AF. Nanoscale reference materials for environmental, health and safety measurements: needs, gaps and opportunities. Nanotoxicol. 2013;7(8):1325–1337. doi: 10.3109/17435390.2012.739664. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu V, Anuradha CV. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Herbs Spices Med Plants. 2007;12(3):13–25. doi: 10.1300/J044v12n03_02. [DOI] [PubMed] [Google Scholar]
- Tripathi DK, Singh S, Singh S, Pandey R, Singh VP, Sharma NC, Prasad SM, Dubey NK, Chauhan DK. An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem. 2017;110:2–12. doi: 10.1016/j.plaphy.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Yanık F, Vardar F. Toxic effects of Aluminum Oxide (Al2O3) nanoparticles on root growth and development in Triticum aestivum. Water Air Soil Pollut. 2015;226(9):296. doi: 10.1007/s11270-015-2566-4. [DOI] [Google Scholar]
- Yanık F, Vardar F. Oxidative stress response to aluminum oxide (Al2O3) nanoparticles in Triticum aestivum. Biologia. 2018;73(2):129–135. doi: 10.2478/s11756-018-0016-7. [DOI] [Google Scholar]
- Zhou P, Su L, Lv A, Wang S, Huang B, An Y. Gene expression analysis of alfalfa seedlings response to acid-aluminum. Int J Genomics. 2016 doi: 10.1155/2016/2095195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuverza-Mena N, Martínez-Fernández D, Du W, Hernandez-Viezcas JA, Bonilla-Bird N, López-Moreno ML, Komárek M, Peralta-Videa JR, Gardea-Torresdey JL. Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses—a review. Plant Physiol Biochem. 2017;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of alumina NPs on the UV absorbance of methanolic extract derived from fenugreek in vitro cultures within 21 days, in comparison with the bulk alumina-treated and control groups. (a) day 1, (b) day 7, (c) day 14, and (d) day 21. (TIFF 11738 kb)