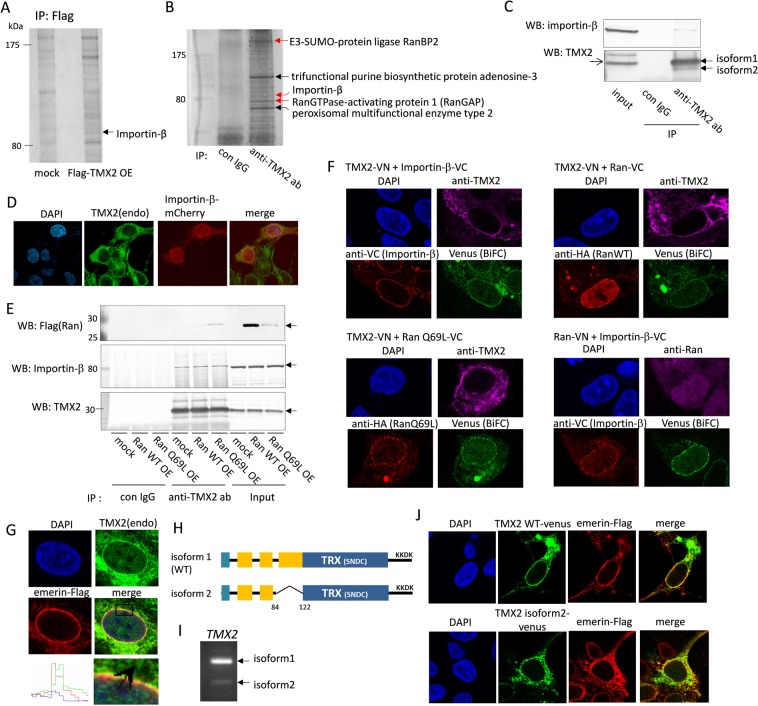
Figure 1.
TMX2 interacts with nuclear import protein. (A) Flag-TMX2 was overexpressed in HEK293 cells and immunoprecipitated with anti-Flag antibody. The band indicated by the arrow was analyzed by LC-MS. (B) Endogenous TMX2-intereacting proteins in HEK293 cells were immunoprecipitated with anti-TMX2 antibody and analyzed by LC-MS. (C) HEK293 cell lysates were immunoprecipitated with anti-TMX2 antibody and analyzed by western blot with anti-importin-β antibody. (D) mCherry-importin-β was expressed in HEK293 cells and immunostained with anti-TMX2 antibody. (E) Flag-Ran WT or -Ran Q69L mutant was overexpressed in cells and immunoprecipitated with anti-TMX2 antibody. (F) TMX2-VN was co-expressed with importin-β-VC, HA-RanWT-VC, or HA-RanQ69L-VC in HEK293 cells, and the overexpression of these proteins were confirmed by anti-TMX2, anti-VC, or anti-HA antibody. Venus fluorescence as BiFC signal indicates the interaction of these fusion proteins. BiFC signal was also detected by co-expression of Ran-VN and importin-β-VC as a positive control. (G) Co-localization of endogenous TMX2 and Flag-emerin was analyzed by immunofluorescence. (H) A diagram of TMX2 WT and isoform 2, which lacks part of the transmembrane region. (I) TMX2 WT and isoform 2 cDNA were amplified by PCR from HEK293 cDNA with the primer sets at 191–507th of TMX2 WT nucleotide. (J) Venus fused with TMX2 WT or isoform 2 was expressed in HEK293 cells, and co-localization with emerin-Flag was analyzed.