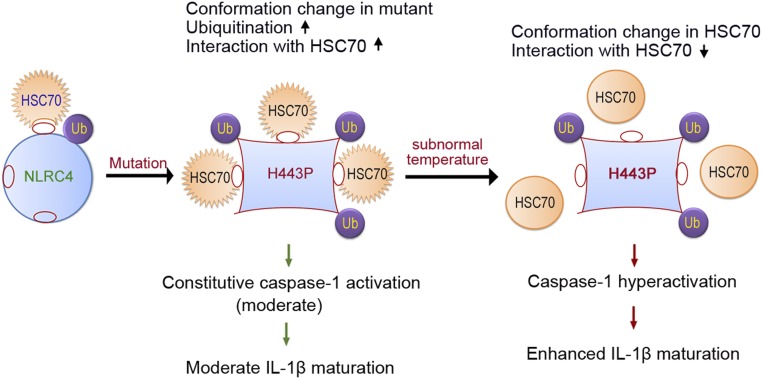
Fig. 7.
Proposed model for regulation of NLRC4-H443P by HSC70 upon exposure to lower temperature. NLRC4 is present in an inactive closed configuration with low levels of ubiquitination (Ub) and weak binding to HSC70. The mutation of H443 to proline causes a conformational change in NLRC4, which enables enhanced ubiquitination and more stable interaction with HSC70. The H443P mutant shows constitutive caspase-1 activation and moderate IL-1β maturation, causing mild inflammation. Upon exposure of cells to subnormal temperature, HSC70 undergoes a conformational change that lowers its ability to interact with H443P. This allows increased ASC-speck formation by H443P, and caspase-1 hyperactivation leading to enhanced IL-1β maturation and hyperinflammation. This mechanism of the differential interaction of HSC70 with H443P in response to subnormal temperature explains the hyperinflammation seen in FCAS patients carrying this mutation.