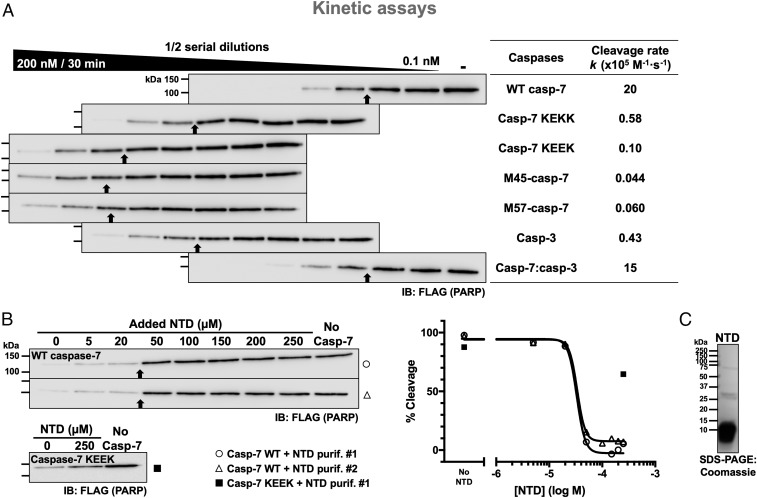
Fig. 1.
Lysine residues 38–41 of caspase-7 promote the cleavage of PARP-1. (A) Efficient cleavage of PARP-1 requires the N-terminal domain. 293C7KO cell extracts expressing full-length FLAG-tagged PARP-1 were incubated with the indicated recombinant caspases at the different concentrations (2-fold serial dilutions of the caspase) for 30 min and analyzed using immunoblotting with an anti-FLAG antibody. The highest and lowest concentrations of caspases are indicated in and over the black wedge, respectively. Immunoblots are aligned to vertically position samples containing the same caspase concentration. The cleavage rates of PARP-1 (k values) are shown in the right-end table for comparison; higher k values signify a faster cleavage rate. As a reference throughout the manuscript, black arrows point to the sample with ∼50% cleavage; thus, the more rightward the arrow, the faster the cleavage. (B) Competition assays showing that the NTD of caspase-7 can prevent PARP-1 cleavage. Samples were treated as in A with a fixed amount of WT caspase-7 (0.78 nM) or caspase-7 KEEK (100 nM) and with varying concentrations of recombinant NTD (a.a. 24–65) from 2 different batches. Quantified data for the inhibition were plotted when possible. (C) Coomassie gel stain showing the integrity of the NTD protein used in B. Black arrows point to the sample with ∼50% cleavage based on densitometry analyses; repeated molecular weight marker values were omitted from subsequent immunoblots.