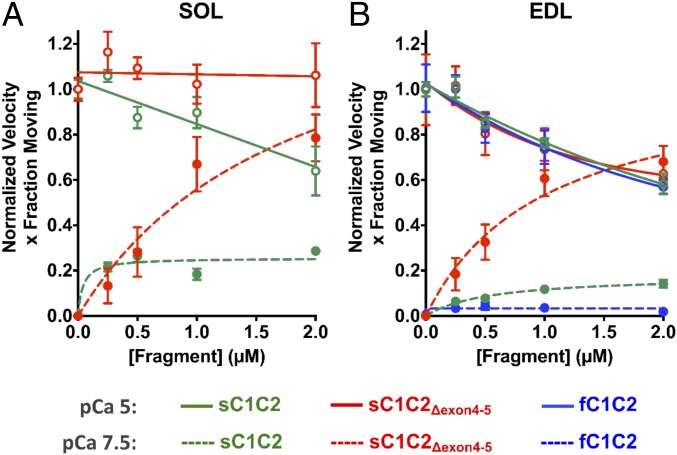
Fig. 5.
Isoform-specific N-terminal MyBP-C fragments differentially modulate SOL and EDL thin filament motility over monomeric myosin in a Ca2+-dependent manner. MyBP-C imparted concentration-dependent (0 to 2 µM) effects on thin filament sliding motility at low (pCa 7.5, closed symbols and dashed lines) and high (pCa 5, open symbols and solid lines) calcium concentrations. Plots are shown as velocity times the fraction of filaments moving, normalized to the value at pCa 5 for each MyBP-C fragment. Data were fitted with noncompetitive inhibition (Ki) and Hill activation (Ka) curves for pCa5 and pCa 7.5, respectively (see SI Appendix, Table S1 for Ki and Ka parameter fits). (A) For SOL thin filament motility at pCa 5, sC1C2 reduced motility by up to 36% with increasing fragment concentration (green solid line). sC1C2Δexon4-5 had negligible impact on thin filament motility (red solid line). At pCa 7.5, sC1C2Δexon4-5 (red dashed line) significantly enhanced thin filament motility, with increasing concentrations compared to sC1C2 (green dashed line). (B) For EDL motility, all 3 N-terminal fragments have comparable inhibitory effects on thin filament velocities at pCa 5 (solid lines). At pCa7.5, fC1C2, sC1C2, and sC1C2Δexon4-5 differentially sensitize the thin filaments to calcium with sC1C2Δexon4-5 (red dashed line) being the greatest sensitizer, compared to sC1C2 (green dashed line) and fC1C2 (blue dashed line). All data are presented as mean ± SEM. See SI Appendix, Fig. S6 for plots of individual velocity and fraction of moving filaments and SI Appendix, Table S1 for parameters of the fits to the data and the number of experiments for each condition.