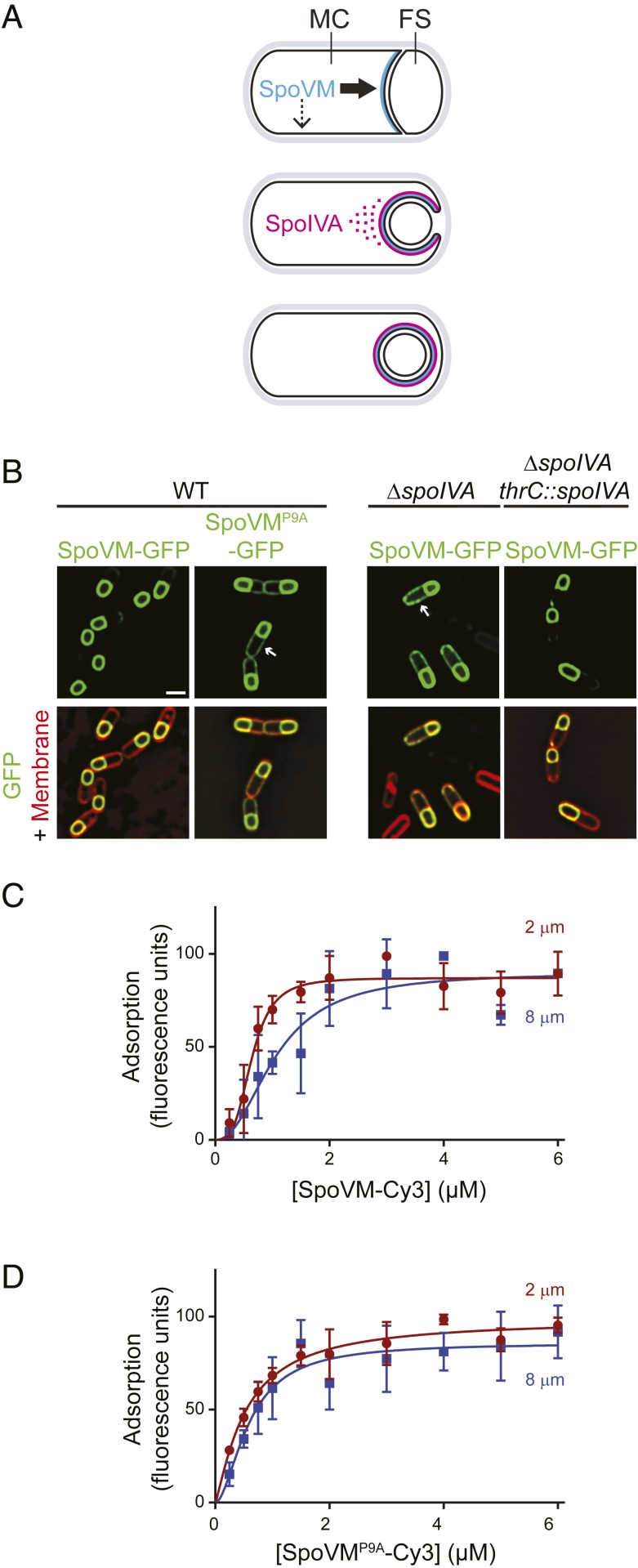
Fig. 1.
Preferential adsorption of SpoVM-Cy3 onto increasingly convex membranes. (A) Schematic of SpoVM and SpoIVA localization within a sporulating B. subtilis cell. Asymmetric division produces a larger mother cell (MC) and a smaller forespore (FS). SpoVM (blue) is produced in the MC and localizes to the engulfing membrane (Top). Thick and thin arrows indicate preferred and less-preferred sites of SpoVM localization, respectively. SpoIVA (magenta) is recruited to the engulfing membrane by SpoVM (Middle). For simplicity, SpoVM and SpoIVA are depicted at different stages of engulfment, but both proteins are produced in the MC after asymmetric division. ATP hydrolysis by SpoIVA drives polymerization on the FS surface (Bottom). (B) Localization of SpoVM-GFP (green; strain CVO1195) or SpoVMP9A-GFP (CVO1395) in wild-type (WT) sporulating B. subtilis cells; SpoVM-GFP localization in the absence of SpoIVA (KR128); or in the absence of SpoIVA complemented with spoIVA expressed from an ectopic chromosomal locus (KR209) 3 h after induction of sporulation. Membranes (red) visualized with FM4-64. Arrows indicate mislocalized GFP signal on the MC membrane. (Scale bar: 1 µm.) (C and D) Adsorption of SpoVM-Cy3 (C) and SpoVMP9A-Cy3 (D) onto differently curved membranes in vitro. Either SpoVM-Cy3 or SpoVMP9A-Cy3 were incubated with a mixed population of 2-μm (maroon) and 8-μm (blue) SSLBs. SSLBs were separated, and fluorescence intensity on each SSLB was measured using flow cytometry. Data were fit with a specific binding with allosteric sigmoidal model: Y = Bmax*Xh/(Kdh + Xh). Data points: median values; error bars: SD (n = ∼20,000).