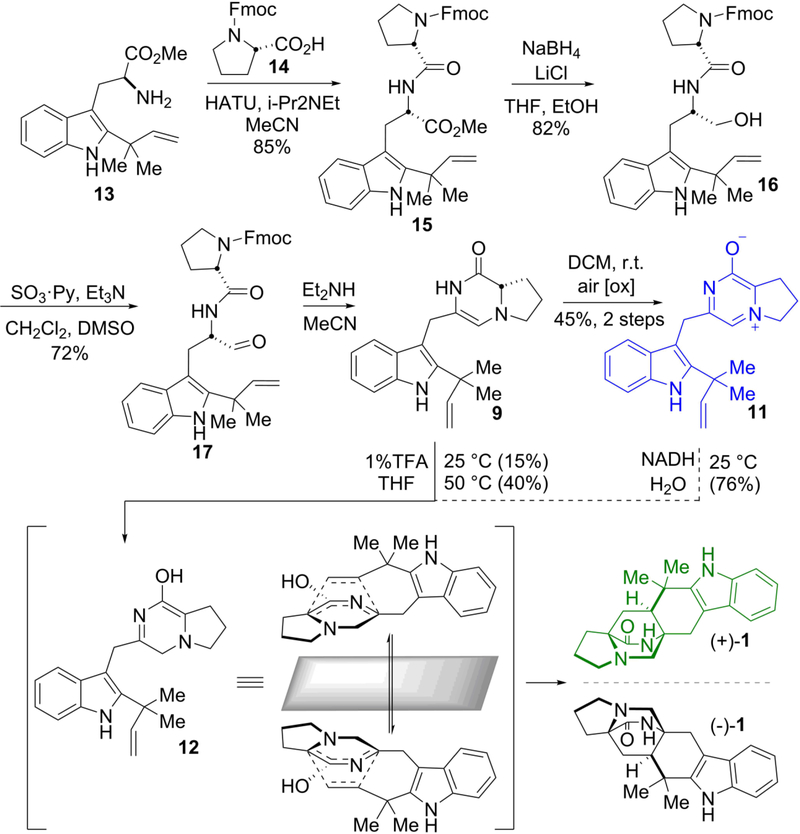
Figure 2. Biomimetic synthesis of premalbrancheamide.
The biomimetic synthesis proceeded through a spontaneous intramolecular [4+2] Diels-Alder reaction from key azadiene intermediate 12 to produce a racemic mixture of syn-premalbrancheamides (1). Zwitterion 11 arises from spontaneous oxidation of 9 and was initially reasoned to be a non-physiological by-product. After discovering that 11 was the preferred substrate of MalC, non-enzymatic chemical reduction by NADH was explored providing 1 in 76% yield from 11. Only optically pure (+)-1 has been isolated from Malbranchea aurantiaca. See SI for complete methods.