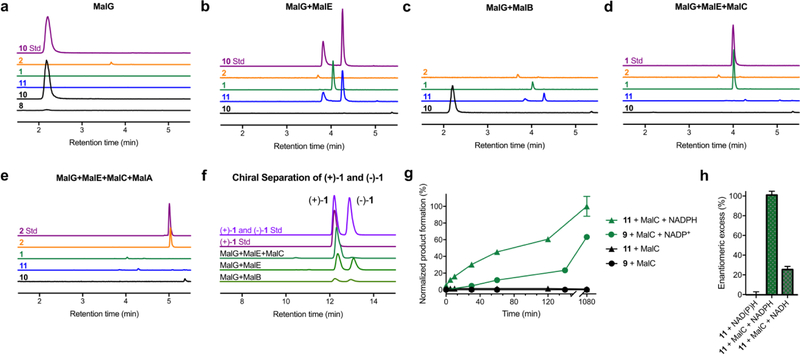
Figure 3. In vitro enzymatic reconstitution of malbrancheamide biosynthesis.
Reactions were monitored by LC/MS. Extracted ion counts (EIC) for key molecules in reaction mixtures are compared to authentic synthetic standards. a. MalG NRPS (excised A1-T1, C, T2, R domains) produced zwitterion 10 by spontaneous oxidation of 8. b – c. Addition of MalE or MalB prenyltransferase formed three products: a prenylated zwitterion 11, and (±)-1. d. MalC Diels-Alderase addition disabled formation of 11 and (−)-1 (see panel f). e. Malbrancheamide 2, the final pathway product, was produced by MalA halogenation of (+)-1. f. Chiral separation of (±)-1 indicates that MalC is an intramolecular [4+2] Diels-Alderase, while neither MalE nor MalB provide enantioselectivity for the spontaneous IMDA reaction. g. MalC-catalyzed reactions under aerobic (11 + MalC) or anaerobic (9 + MalC) conditions. The aerobic route with 11 as the pathway intermediate was more efficient than the anaerobic route from 9. h. Effect of cofactor on the enantiomeric excess of the MalC-catalyzed Diels-Alder reaction. MalC provided limited enantioselectivity when NADH was used as cofactor. EIC traces are colored by compound as in Figure 1b, authentic standards are in purple or pink. For panel g and h, all data represent the average of triplicate independent experiments (center values, mean; error bars, SD; n = 3).