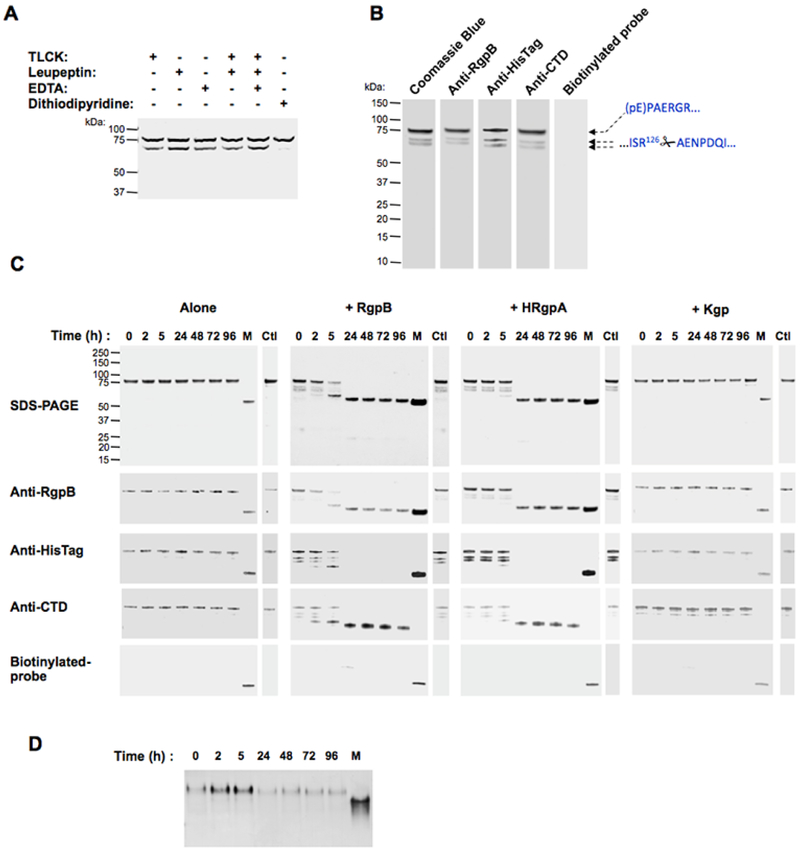
Figure 2. Purified proRgpB6His undergoes partial autoproteolytic processing with limited increase in enzymatic activity.
ProRgpB6His (0.4 mg/ml) was incubated at room temperature in gingipain activity buffer with/without 5 mM DTT supplementation. At various time points, aliquots were removed and subjected to (A) SDS-PAGE electrophoresis, or (B) Western blot analysis with pAb anti-RgpB, mAb anti-6HisTag and pAb anti-CTD domain. (C) Active-site labelling using the biotinylated probe BiRK to detect active forms of partially processed proRgpB. Mature RgpB bearing a C-terminal HisTag (Veillard et al, 2015) was used as a control (lane M). (D) The activity of RgpB at each time point was determined with the chromogenic substrate L-BAPNA. Results are expressed as percentage of activity of the native RgpB incubated in the same condition (n = 3).