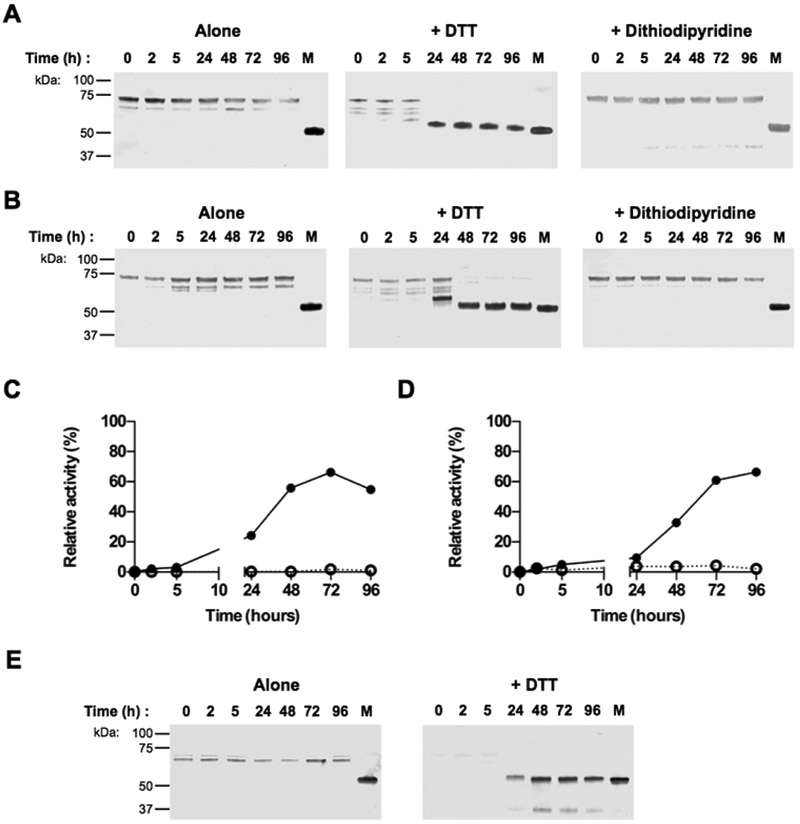
Figure 6. ProRgpB6His is proteolytically processed by whole cell extracts or intact bacterial cells in gingipain-null P. gingivalis strain.
ProRgpB6His (0.4 mg/ml) was incubated with the (A) whole cell extract (WCE) of P. gingivalis gingipain-deficient strain ΔK/ΔRAB-A or (B) with the whole bacteria diluted in RPMI media (B-RPMI), alone or in the presence of DTT (5 mM) or 4,4’-Dithiodipyridine disulphide (2 mM). At various time points, aliquots were removed to follow the maturation of the zymogen by Western blotting with pAb anti-RgpB. RgpB activity was also recorded during incubation with (black circle) or without DTT (open circle) using the chromogenic substrate L-BAPNA (panel C: WCE; panel D: intact bacterial cells in B-RPMI). Results are expressed as percentage of activity of the mature RgpB incubated under the same condition (n = 3). (E) The active form of RgpB obtained during the incubation with the bacterial suspension was identified by active-site labelling using the biotinylated probe BiRK. Mature RgpB bearing a C-terminal HisTag (lane M) was used as control.