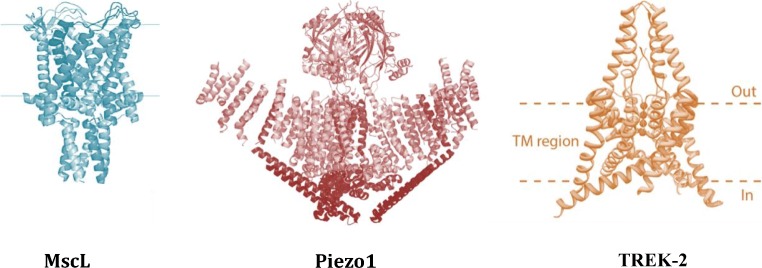
Fig. 1.
Examples of truly mechanically activated ion channels. MscL, Piezo1, and TREK-2 are activated and controlled by mechanical force over their whole dynamic range from the fully closed to the fully open state. The 3D X-ray structure of MscL from Mycobacterium tuberculosis shows the channel homopentamer in the closed state (adapted from Chang et al. (1998)). The Piezo1 structure determined by cryo-EM shows the first low-resolution structure of the trimeric channel in its closed conformation (adopted from Ge et al. (2015)). The higher resolution structure has later been determined by several laboratories (Guo 2017; Zhao et al. 2018; Saotome et al. 2018). Crystal structure of the human TREK-2 channel, a member of the K2P family which forms dimers. Each monomer adds two-pore loops to the structure to end up with pseudo-tetrameric assembly (Dong et al. 2015). One of the structural characteristics of the truly mechanically gated channels is recently resolved lipid-binding domains, similar to the MscL N-terminus (Bavi et al. 2016). Such lipid-binding structural domains were identified in the MscS-like channels, K2P family of channels (TREK/TRAAK), and most recently Piezo1 ion channels (Bavi et al. 2017a, b)