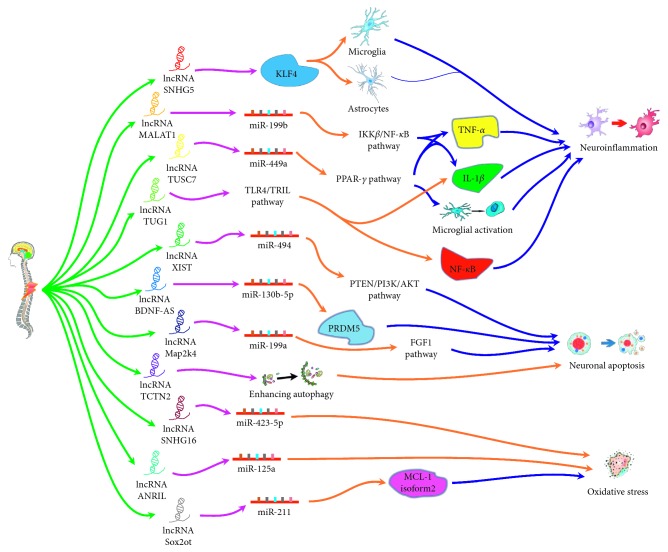
Figure 1.
The probable mechanisms of the effects produced by lncRNAs on SCI. There are several lncRNAs that have been reported to play different roles mainly in the process of neuroinflammation, neuronal apoptosis, and oxidative stress following SCI. Firstly, lncRNA SNHG5 was demonstrated to increase the viability of microglia and astrocytes via upregulating KLF4. lncRNA MALAT1 overexpression could cause the activation of IKKβ/NF-κB signaling pathway via the modulation of a miR-199b and increased proinflammatory cytokines. Overexpression of lncRNA TUSC7 could inhibit microglial activation and the expression of inflammatory factors in microglia cells by regulating PPAR-γ through miR-449a. Downregulation of lncRNA TUG1 inhibited TLR4 signaling pathway-mediated inflammatory damage via suppressing TRIL expression. Secondly, downregulation of lncRNA XIST contributed to the limiting neuronal apoptosis via as a sponge for miR-494 which ultimately regulated PTEN/PI3K/AKT signaling pathway. Knockdown of lncRNA BDNF-AS was identified to suppress neuronal cell apoptosis by sponging miR-130b-5p and targeting gene PRDM5. Overexpression of lncRNA Map2k4 and lncRNA TCTN2 could reduce apoptosis after SCI by regulating a miR-199a/FGF1 pathway and enhancing cell autophagy, respectively. Thirdly, overexpressed lncRNA SNHG16 could alleviate H2O2-induced cell injury by mediating miR-423-5p in PC-12 cells. Suppression of lncRNA ANRIL might aggravate H2O2-induced injury in PC-12 cells by targeting miR-125a. However, downregulation of lncRNA Sox2ot might relieve H2O2 injury in PC-12 cells via regulating the miR-21-myeloid cell leukemia-1 isoform2 axis.