Figure 1, Key Figure. Priming and maintenance of T. cruzi CD8+ T cell response.
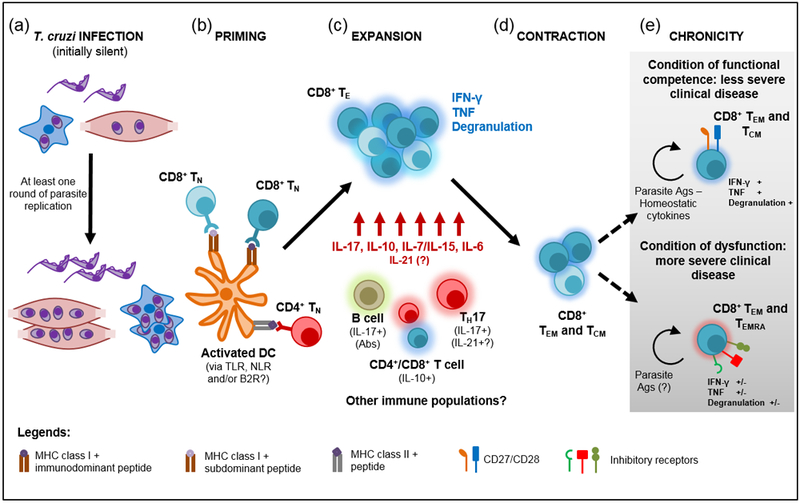
a) T. cruzi infects different cell types within the host but at least one round of parasite replication is necessary to allow the accumulation of sufficient amounts of parasite antigens (Ags) and ligands able to activate antigen presenting cells with T cell priming ability. Dendritic cells (DC) recognize T. cruzi through ligation of toll-like receptors (TLR) that are nevertheless not essential for CD8+ T cell priming. Alternatively, Nod-like receptors (NLR) or bradykinin 2 receptors (B2R) may be involved in this process. b) Activated DCs prime naïve CD8+ T cells (TN) that recognizes immunodominant as well as subdominant parasite epitopes. CD4+ T cell help seems to be mainly required in the induction of CD8+ T cells specific for immunodominant peptides. c) Upon priming, there is a robust expansion of parasite-specific effector CD8+ T cells (TE) that show polyfunctional effector response and are critical for the control of parasite replication. TE CD8+ T cell survival and effector function is sustained by particular cytokines and soluble mediators produced by different cell subsets. d) After the limitation of parasite burden, the CD8+ T cell response contracts and gives rise to effector memory (TEM) and central memory (TCM) CD8+ T cells that persist during the chronic phase. e) Although memory CD8+ T cells remains functional to limit parasite outgrowth, at least two scenarios have been reported in the chronic phase in relation to CD8+ T cell phenotype and functional competence.
