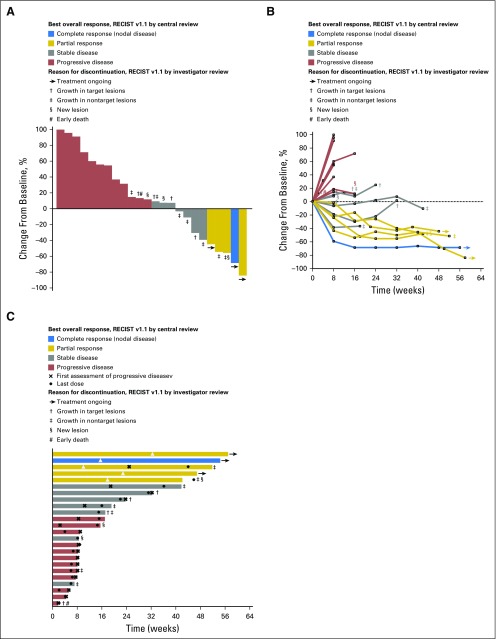
Fig 1.
Antitumor activity of pembrolizumab based on RECIST v1.1 assessed by central review. (A) Best percentage change from baseline in the sum of the longest diameters of target lesions. (B) Longitudinal change from baseline in the sum of the longest diameters of target lesions. (C) Time to and durability of response. For each panel, only patients who received at least one pembrolizumab dose and who had evaluable tumor measurements based on RECIST v1.1 assessed by central review at baseline and at least one postbaseline assessment were included (n = 24). Reasons for treatment discontinuation in patients whose change from baseline per RECIST v1.1 by central review was ≤ 20% are indicated; because patients were managed by investigator assessment, reasons for discontinuation are based on RECIST v1.1 by investigator review. In panel C, length of bars equals time to last imaging assessment by central review. RECIST, Response Evaluation Criteria in Solid Tumors.