Abstract
Purpose of review
This review will summarize recent findings of the effect of supplemental fatty acids, with an emphasis on omega-3 polyunsaturated fatty acids, as a treatment for diabetic peripheral neuropathy.
Recent findings
Pre-clinical studies have provided evidence that treating diabetic rodents with δ linolenic acid (omega-6 18:3) and to a greater extent with eicosapentaenoic and docosahexaenoic acids (omega-3 20:5 and 22:6, respectively) improve and even reverse vascular and neural deficits. Additional studies have shown resolvins, metabolites of eicosapentaenoic and docosahexaenoic acids, can induce neurite outgrowth in neuron cultures and that treating type 1 or type 2 diabetic mice with resolvin D1 or E1 provides benefit for peripheral neuropathy similar to fish oil.
Summary
Omega-3 polyunsaturated fatty acids derived from fish oil and their derivatives have anti-inflammatory properties and could provide benefit for diabetic peripheral neuropathy. However, clinical trials are needed to determine whether this statement is true.
Keywords: Diabetic peripheral neuropathy, Omega-3 polyunsaturated fatty acids, Omega-6 polyunsaturated fatty acids, Resolvin, Inflammatory stress, Oxidative stress
Introduction
Peripheral neuropathy is the most common of diabetic complications affecting about 50% of subjects with either type 1 or type 2 diabetes [1••, 2••, 3••]. It drastically reduces the quality of life and is the leading cause of non-trauma related amputations of lower limbs [4, 5]. The 5-year survival rate for patients after non-trauma related amputation is estimated at less than 50% [4]. About 25% of subjects with diabetic neuropathy experience pain and treatments exist to help alleviate this symptom; how-ever, there is no treatment for repair of nerve damage other than good glycemic control, which only delays its progression in subjects with type 1 diabetes and has little impact in subjects with type 2 diabetes [2••, 5–8]. The mechanisms responsible for diabetic neuropathy and potential treatments have been the subject of intense research for many years. Studies with animal models have identified a wide range of therapeutic targets for treatment of diabetic peripheral neuropathy, but translation to human subjects has not been successful [1••, 9, 10•, 11, 12••, 13–19]. There are many explanations that may account for the lack of success in discovering a treatment(s) for diabetic neuropathy [20, 21••]. Nonetheless, the severity of this problem necessitates a continued pursuit for a treatment. In this review, article study of the potential role for dietary fatty acids as a contributor as well as a treatment for diabetic peripheral neuropathy will be presented with an emphasis on omega-3 poly-unsaturated fatty acids commonly found in fish oil and other cold water marine mammals.
Dietary Lipid Contribution to Peripheral Neuropathy
Abnormal lipid metabolism or excess lipids have been associated with a wide variety of diseases including diabetic neuropathy. High-fat diets consisting primarily of saturated fat derived from lard have been used to induce dietary obesity in rodent models. Studies from my laboratory has shown that feeding rodents a high-fat diet causes a slowing of sensory nerve conduction velocity, decrease in intraepidermal and corneal nerve fibers, and abnormal sensory nerve perception as indicated by increased thermal latency and reduced cornea sensitivity and in rats vascular dysfunction in epineurial arterioles of the sciatic nerve [22–26]. Interestingly, we have not been able to detect a slowing of motor nerve conduction velocity in rodents fed a high-fat diet unless they have been on such a diet for almost a year. This suggests that there may be a difference in the pathogenesis caused by excess saturated fats in the diet on large and small or myelinated and un-myelinated nerve fibers. Other laboratories have reported similar events overall when feeding rodents a high fat/Western diet. Obrosova et al. demonstrated that female C57Bl6/J mice fed a high-fat diet for 16 weeks developed obesity, increased plasma free fatty acid and insulin levels, and impaired glucose tolerance [27]. In these studies, they found a slowing of both motor and sensory nerve conduction velocities, tactile allodynia, and thermal hypoalgesia in the absence of intraepidermal nerve fiber loss or axonal atrophy. Two other studies using C57Bl6/J mice fed a high-fat diet for 14–16 weeks found impaired glucose tolerance and reduction in motor and sensory nerve conduction velocity and decreased intraepidermal nerve fiber density [28, 29]. In addition, in the study by Hinder et al. [28], they demonstrated that reversing the diet of high-fat fed mice to a normal diet for 6 weeks improved metabolic parameters and nerve function. We performed a similar reversal study in high-fat fed Sprague-Dawley rats and obtained mixed results [22]. Rats were fed a high-fat diet for 12 weeks and then a normal diet for 12 weeks. Reversing the diet improved glucose utilization and steatosis but only marginally improved neuropathology. We concluded that more extensive treatment or a longer duration is required to reverse peripheral neuropathy in obese rats. In another study, Ozay et al. [30] fed male Wistar rats a high-fat diet for 12 weeks and reported that nerve fiber diameter and thickness of the myelin sheath were significantly lower in the high-fat fed rats.
Besides animal studies, there is evidence that suggests development of neuropathy in human subjects with impaired glucose tolerance and metabolic syndrome [31•]. Early onset of neuropathy in patients with pre-diabetes is frequently reported to be a burning pain and allodynia and loss of small myelinated Aδ fibers or unmyelinated C fibers [28]. In the general population, the prevalence of neuropathy in prediabetes is intermediate and milder between overt diabetes and subjects with normoglycemia [32]. Above results have led to studies examining the effect of lipid lowering on diabetic neuropathy. In type 2 diabetic mice, treatment with fenofibrate ameliorated endothelial and neural damage by activating the peroxisome proliferative-activated receptor-α [33]. Studies with fenofibrate were shown to lower levels of atypical sphingolipids, which have been linked to inherited forms of sensory neuropathy, in plasma of dyslipidemic patients, and the authors proposed that this could be a novel approach to prevent/treat diabetic neuropathy [34]. Fenofibrate has also been shown to improve microvascular complications in human subjects with type 2 diabetes [35]. A study by Davis et al. using a large observational cohort, termed the Freemantle Diabetes Study, suggested that the use of statins or a fibrate may slow the progression of neuropathy in patients with type 2 diabetes [36]. In a multi-center study referred to as the Fenofibrate and Event-Lowering in Diabetes (FIELD) study conducted using 9795 patients with type 2 diabetes randomized to placebo or fenofibrate reported a significantly lower rate of non-traumatic amputations in the fenofibrate-treated group [37]. A randomized trial enrolled 10,251 participants with type 2 diabetes and targeted to receive either intensive or standard treatment for glycemia (target glycated hemoglobin level, < 6.0 or 7.0 to 7.9%, respectively) and also for dyslipidemia (160 mg daily of fenofibrate plus simvastatin or placebo plus simvastatin) or for systolic blood pressure control (target, < 120 or < 140 mmHg). A subgroup of 2856 participants was evaluated for the effects of these interventions at 4 years on the progression of diabetic retinopathy by three or more steps on the Early Treatment Diabetic Retinopathy Study Severity Scale. It was found that intensive glycemic control and intensive combination treatment of dyslipidemia, but not intensive blood-pressure control, reduced the rate of progression of diabetic retinopathy [38]. Overall, a number of clinical studies have led investigators and clinicians to conclude that hyperglycemia is not the only factor contributing to diabetic neuropathy, particularly in patients with type 2 diabetes, and that components of the metabolic syndrome, such dyslipidemia, obesity, insulin resistance, and cardiovascular risk factors including hypertension, may also have a contributing role [39, 40]. For reasons that are not entirely clear, lipid lowering drugs are not commonly recognized as a treatment for diabetic neuropathy.
Lipids as Potential Therapy for Peripheral Neuropathy?
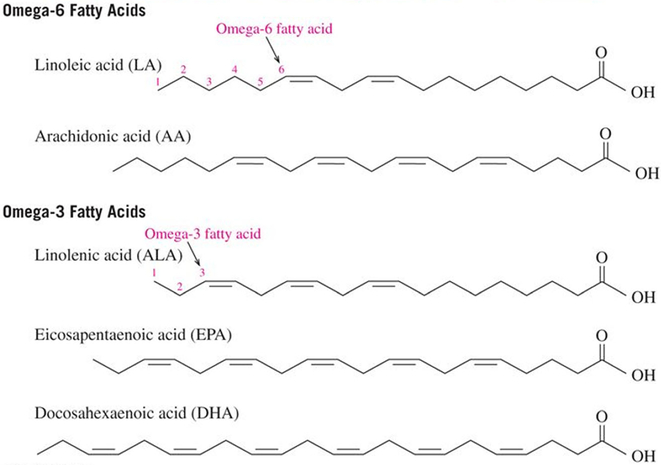
Lipids have many roles in the mammalian system ranging from structural components of membranes to signaling molecules. In the section above, we have seen that in excess lipids in the form of saturated fatty acids can contribute to development and progression of peripheral neuropathy. However, is it possible that lipids could also have a role in the treatment of peripheral neuropathy? Besides saturated fatty acids, which have no double bonds, fatty acids may also have a single double bond with oleic acid (18:1) being the most common fatty acid of this class or they may have multiple double bonds and referred to as polyunsaturated fatty acids. There are two classes of polyunsaturated fatty acids commonly referred to as omega-6 or omega-3 (Fig. .1). Omega-6 fatty acids (also referred to as ω−6 fatty acids or n-6 fatty acids) are a family of polyunsaturated fatty acids that have in common a final carbon-carbon double bond in the n-6 position, that is, the sixth bond, counting from the methyl end. Linoleic acid (18:2) is the shortest-chained omega-6 fatty acid, and arachidonic acid (20:4) is the most common and important for synthesis of signaling molecules. Both omega-6 and omega-3 are categorized as essential fatty acids because the human body is unable to synthesize them. In omega-3 fatty acids (also referred to as ω−3 fatty acids or n-3 fatty acids), the first double bond is between the third and fourth carbon atoms from the methyl end of the carbon chain. The three primary omega-3 fatty acids involved in human physiology are α-linolenic acid (18:3), found in plant oils, and eicosapentaenoic acid (20:5) and docosahexaenoic acid (22:6), both commonly found in fish/marine oils.
Fig.1.
Structure of the primary omega-6 and omega-3 polyunsaturated fatty acids
Mammals synthesize the longer chain polyunsaturated fatty acids from linoleic acid and α-linolenic acid, which are the two precursors of omega-6 and omega-3 fatty acid families provided by the diet [41]. Specific enzymes, desaturases and elongases, are involved in this pathway, but the conversion of precursors to long chain polyunsaturated fatty acids is generally low in humans. In addition, diabetes impairs the desaturases activities [41, 42]. Consequently, the decrease in bioavailability of polyunsaturated fatty acids affects the fatty acid composition of membrane phospholipids and signaling pathways [41]. The National Health and Nutrition Examination Survey (NHANES) reported that the mean dietary intake of γ-linolenic acid (18:3, omega-6) was significantly lower among adults with peripheral neuropathy compared to those patients without peripheral neural neuropathy [43]. This suggests that increasing the intake of polyunsaturated fatty acids may be an effective treatment to delay or prevent development of diabetic peripheral neuropathy.
Omega-6 and omega-3 polyunsaturated fatty acids have been tested pre-clinically as a potential treatment for diabetic peripheral neuropathy. The effect of γ-linolenic acid or evening primrose oil (natural oil enriched in γ-linolenic acid) on neuropathy was first examined nearly 30 years ago in diabetic animal models. Tomlinson et al. reported that treating type 1 diabetic rats with evening primrose oil prevented completely the development of motor nerve conduction deficit [44]. Cameron et al. also reported that treating type 1 diabetic rats with Efamol Marine for 2 months increased plasma levels of γ-linolenic acid and prevented nerve conduction velocity deficits and improved nerve hypoxia [45]. Other investigators have conducted similar experiments and have shown that treating diabetic rats with γ-linolenic acid improved neuropathy and membrane composition of the sciatic nerve and Na+, K+ ATPase activity [46, 47]. Investigators have also examined the effect on diabetic neuropathy in pre-clinical studies of the combination of γ-linolenic acid and α-lipoic acid. α-Lipoic acid is an antioxidant that is available over the counter. Two groups reported that a lipoic acid/γ-linolenic acid conjugate was effective against multiple indices of experimental diabetic neuropathy [48, 49]. Both groups reported that the combination of α-lipoic acid and γ-linolenic acid was more effective than monotherapy in improving electrophysiological and neurochemical endpoints. Investigators have also examined the combined effect of α-lipoic acid and evening primrose oil on diabetic neuropathy [50, 51]. These studies have demonstrated that this combination was effective at treating diabetes-induced changes in enteric nerves of the rat ileum and improving endoneurial blood flow. Two clinical studies have examined the effect of γ-linolenic acid on human diabetic peripheral neuropathy. Jamal and Carmichael studied 22 patients with distal diabetic polyneuropathy using a double-blind, placebo-controlled study design to assess the effect of dietary supplementation of γ-linolenic acid on their neuropathy [52]. Patients received capsules containing 360 mg of γ-linolenic acid or indistinguishable placebo daily for 6 months. Compared to the placebo group, the patients on γ-linolenic acid showed a significant improvement in neuropathy symptom scores as well as a number of electrophysiology endpoints [52]. In the γ-Linolenic Acid Multicenter Trial, 111 patients with mild diabetic neuropathy were randomized into a double-blind, placebo-controlled study using γ-linolenic acid at a dose of 480 mg per day [53]. The investigation included 16 parameters and at the end of the study the response to γ-linolenic acid was more favorable to placebo for 13 parameters and statistically significant. Overall, these studies would suggest that γ-linolenic acid with or without α-lipoic acid could be an effective treatment for diabetic peripheral neuropathy. However, the use of γ-linolenic acid has not been an accepted therapeutic approach.
Fish oil has been the most common source for omega-3 polyunsaturated fatty acids and is enriched in eicosapentaenoic and docosahexaenoic acids. Docosahexaenoic acid is the most abundant omega-3 polyunsaturated fatty acid in the mammalian brain and retina, and up to 60% of all fatty acids esterified in neuronal plasma membrane phospholipids at the sn-2 position of the glycerol moiety consist of docosahexaenoic acid [54, 55]. Even though the brain has a high concentration of docosahexaenoic acid, it has a limited capacity to synthesize it from α-linolenic acid suggesting that docosahexaenoic acid required by the brain is taken up from the circulation [54].
Work from my laboratory has demonstrated that enriching the diet of chronic diabetic mice or rats with menhaden (fish) oil can not only slow progression of diabetic peripheral neuropathy as determined by protection of motor and sensory nerve conduction velocity and prevention of thermal hypoalgesia but also can stimulate reversal and repopulation of sensory nerves in the skin and cornea [22, 56–59, 60••, 61]. Different cellular properties and functions may be altered when cells are enriched in long chain polyunsaturated fatty acids such as membrane fluidity, which may alter carriermediated transport, the properties of certain membrane-bound enzymes, binding to the insulin and opiate receptors, phagocytosis, endocytosis, depolarization-dependent exocytosis, immunologic and chemotherapeutic cytotoxicity, prostaglandin production, and cell growth [62]. However, in our studies with menhaden oil we have attributed the beneficial effects on diabetic peripheral neuropathy to the reduction of oxidative and inflammatory stress and the neuroprotective properties of resolvins [63]. Focusing on oxidative stress and inflammation as a therapeutic target for diabetic peripheral neuropathy is not novel since other studies have also concluded that these pathways to be important [10•, 64, 65].
Other laboratories besides our own have reported favorable results when treating diabetic rodents with omega-3 polyunsaturated fatty acids for neuropathy related endpoints. Gerbi et al. reported that fish oil supplementation prevented decrease in Na+, K+ ATPase activity in the sciatic nerve, diabetes-induced slowing of nerve conduction velocity, and neuroanatomical changes in rats [66]. Julu reported that eicosapentaenoic acid prevented slowing of nerve conduction velocity in streptozotocin-induced diabetic rats [67]. Two recent studies have suggested that treatment with fish oils may also be beneficial in preventing painful diabetic neuropathy. Heng et al. reported that dietary supplementation of docosahexaenoic acid inhibited mechanical allodynia and thermal hyperalgesia by decreasing the excitability of dorsal root ganglions [68]. Li et al. reported that supplementing the diet of diabetic rats with fish oil prevented mechanical allodynia and thermal hyperalgesia by blocking nuclear factor-κB-mediated inflammatory pathways [69].
In a clinical study conducted over 20 years ago, Okuda et al. reported that treating patients with type 2 diabetes with a new, highly purified, ethyl esterification product from natural eicosapentaenoic acid improved the clinical symptom (cold-ness, numbness) as well as the vibration perception threshold sense of the lower extremities [70]. They also reported a significant decrease of serum triglycerides and significant de-crease of the excretion of albumin in urine. They concluded that omega-3 polyunsaturated fatty acids have significant beneficial effects on diabetic neuropathy and serum lipids as well as other diabetic complications such as nephropathy. In a more recent study, Lewis et al. reported that treating 40 type 1 diabetic patients with omega-3 polyunsaturated fatty acids derived from seal oil for 1 year increased corneal nerve fiber length by 29% but did not change nerve conduction or sensory function [71••]. These are encouraging results since studies in the last few years have promoted changes in corneal nerve fiber density as a potential early marker for diabetic neuropathy [72–74]. It will be interesting to see if a longer treatment phase may lead to improvement in other neurological deficits.
As mentioned above, the focus of our studies of the potential mechanism for the effect of eicosapentaenoic and docosahexaenoic acids on diabetic peripheral neuropathy has been on the effect of resolvins. Resolvins are oxygenated metabolites of eicosapentaenoic acid (E series resolvins) and docosahexaenoic acid (D series resolvins) and are generated through series of reactions that include the enzyme 15-lipoxygenase-1. Docosahexaenoic acid can also be converted into neuroprotectin-1. Eicosapentaenoic and docosahexaenoic acids are excellent substrates for 15-lipoxygenase-1, and resolvin formation is elevated by consuming increased amounts of eicosapentaenoic and docosahexaenoic acids as occurs when increasing intake of fish oils [75, 76]. It has been shown that regeneration of corneal nerves damaged by refractive surgery can be increased by treatment with docosahexaenoic acid through synthesis of neuroprotectin-1 [77, 78]. This group also reported that neuroprotectin-1 increases neurite outgrowth from trigeminal ganglia neurons from Swiss Webster mice [77]. Robson, et al. reported that omega-3 fatty acids promote neurite outgrowth from dorsal root ganglia neurons, and the effect of docosahexaenoic acid was still prominent in neurons from aged animals [79]. We have obtained similar results when we treated dorsal root ganglion with resolvin D1 [58]. In a recent study, we used exogenous injections of resolvin D1, E1, and methyl esters of resolvins D1 or D2 and compared their effect to dietary enrichment with menhaden oil on neuropathic endpoints in type 2 diabetic mice [80]. As stated above, we have previously demonstrated in both type 1 and type 2 diabetic mice that endogenous treatment with daily injections of resolvin D1 was able to improve many endpoints associated with diabetic peripheral neuropathy [58, 60••]. The improvement observed with treatment of resolvin D1 was similar to the improvement we obtained through enriching the diet with menhaden oil [58, 60••]. Resolvin D1 levels in serum was also found to increase in the mice treated with diet enriched in menhaden oil [58, 60••]. In our most recent study, we were interested in comparing the effect of resolvin D1, derived from docosahexaenoic acid, to resolvin E1, derived from eicosapentaenoic acid, on neuropathic endpoints as well as to determine if the methyl ester analogues of resolvins D1 or D2 are more potent than resolvin D1 due to a reported longer circulatory half-life for these analogues [80•]. Results from this study indicated that untreated diabetic mice had mechanical allodynia, were thermal hypoalgesic, and had reduced motor and sensory nerve conduction velocities, and innervation of the cornea and skin was decreased. Treating diabetic mice with daily injections of resolvin D1 or E1 improved these neurological endpoints similar to dietary treatment with menhaden. However, treating the diabetic mice with the methyl esters of resolvins D1 or D2 was generally less potent than menhaden oil or resolvins D1 or E1. The two important findings from this study were that resolvins derived from eicosapentaenoic acid or docosahexaenoic acid were equally effective in improving neuropathy and that methyl esters of resolvins D1 or D2 even though they may have longer half-lives were not as efficacious as resolvin D1. Overall, this study provided further support for omega-3 polyunsaturated fatty acids derived from fish oil via in part due to their metabolites could be an effective treatment for diabetic neuropathy. Resolvins as mediators of inflammation have been shown to have multiple effects on other tissues as previously reviewed [81••].
In another study, previously unreported, we examined the effect of resolvin D1 and menhaden oil on diabetic neuropathy in female C57Bl6/J mice. We had previously demonstrated that inducing type 2 diabetes through a combination of high-fat diet and streptozotocin and the development of peripheral neuropathy is similar for male and female C57Bl6/J mice [82]. In this study, after 6 weeks of diabetes, female mice were treated for 8 weeks with menhaden (fish) oil enriched diet or with daily injections of resolvin D1. Afterwards, multiple neuropathies related endpoints were examined (Table 1). Diabetic mice were hyperglycemic compared to control mice and treatment with menhaden oil or resolvin D1 did not improve blood glucose levels. Motor and sensory nerve conduction velocity was significantly decreased by diabetes and both were significantly improved with treatment. Intraepidermal nerve fiber and corneal nerve fiber densities were significantly decreased in diabetic mice and improved with the menhaden oil-enriched diet or exogenous resolvin treatment. Two behavioral studies were performed relating to sensory nerve sensitivity. Diabetic female mice demonstrated latency to a thermal challenge and greater sensitivity to a mechanical force. Both of these endpoints were significantly improved with menhaden oil or resolvin D1 treatments. This study further demonstrated that fish oil is an effective treatment for multiple endpoints related to diabetic neuropathy and that the beneficial effects are not gender specific.
Table 1.
Effect of menhaden oil dietary enrichment or daily treatment with resolvin D1 of diabetic female mice on fasting blood glucose, motor and sensory nerve conduction velocity (MNCV/SNCV), intraepidermal nerve fiber density (IENF), cornea nerve fiber length, thermal nociception, and mechanical allodynia
| Determination | Control | Diabetic | Diabetic + menhaden oil | Diabetic + resolvin D1 |
|---|---|---|---|---|
| Fasting blood glucose (mg/dl) | 204 ± 7 | 407 ± 25a | 343 ± 30a | 375 ±31a |
| MNCV (m/s) | 34.9 ± 1.3 | 21.9 ± 0.9a | 31.9 ± 1.2b | 31.3 ± 1.4b |
| SNCV (m/s) | 17.9 ± 0.2 | 15.0 ± 0.5a | 17.9 ± 0.2b | 18.2 ± 0.3b |
| IENF (profiles/mm) | 24.9 ± 0.5 | 15.7 ± 0.3a | 21.5 ± 0.a,b | 20.8 ± 0.3a,b |
| Corneal nerve fiber length (mm/mm2) | 2.00 ± 0.11 | 0.93 ± 0.07a | 1.81 ±0.15b | 1.70 ± 0.14b |
| Thermal nociception (s) | 4.94 ± 0.07 | 7.25 ± 0.13a | 5.41 ± 0.16b | 5.87 ± 0.14a,b |
| Mechanical allodynia (g) | 2.82 ± 0.08 | 1.15 ± 0.03a | 1.82 ± 0.09a,b | 1.69 ± 0.10a,b |
Data are presented as the mean ± S.E.M
P < 0.05 compared to control
P < 0.05 compared to diabetic. Number of experimental animals for each group was 15
If the pro-resolving lipid mediators of eicosapentaenoic acid and docosahexaenoic acid are potential treatments for peripheral diabetic neuropathy, then discovering means to naturally increase their production in vivo may promote greater benefits. Aspirin is one compound that has been reported to increase the production of resolvins [83]. However, aspirin has side effects that make it undesirable for long term clinical use. Another compound with aspirin-like properties that has been reported to provide beneficial effects in obesity and diabetes by targeting established chronic inflammatory signaling and reducing insulin resistance is salsalate; aspirin and salsalate in vivo are metabolized to salicylate [83]. Salsalate activates brown adipose tissue in high-fat fed mice and improves glucose tolerance [84]. In human subjects with diabetes, salsalate improved insulin resistance and the lipid, lipoprotein, and apoprotein profile of insulin-resistant individuals who were overweight or obese [85–88]. Based upon studies utilizing salsalate, one group concluded that targeting inflammation and nuclear factor-κB may be a viable therapeutic approach for treating type 2 diabetes [89]. We recently completed a study designed to examine the efficacy of fish oil with or without salsalate on vascular and neural complications using a type 2 diabetic rat model [90]. Four weeks after the onset of hyperglycemia diabetic rats was treated via the diet with three different amounts of menhaden oil (10, 25, or 45% kcal) with or without salsalate for 12 weeks. Afterwards, vascular reactivity of epineurial arterioles and neuropathy-related endpoints were examined. The addition of salsalate to high-fat diets enriched with 10 or 25% kcal of menhaden oil protected vascular reactivity to acetylcholine and calcium gene-related peptide, motor and sensory nerve conduction velocity, thermal nociception, intraepidermal nerve fiber density, and cornea sensitivity to a greater extent than 10 or 25% menhaden oil alone. Vascular and neural function was maximally protected with diet containing 45% kcal as menhaden oil and adding salsalate did not provide any additional benefit. Salsalate alone in the high-fat diet of diabetic rats provided minimal protection/improvement of vascular and neural dysfunction. Adding salsalate to the menhaden oil enriched diets increased serum levels of resolvin D1. These studies imply that dietary salsalate in combination with lower amounts of menhaden oil can provide greater benefit toward diabetes-induced vascular and neural impairment than menhaden oil alone. Currently, a phase 2/3 human clinical trial evaluating salsalate for diabetic neuropathy is ongoing (NCT ).
Conclusion
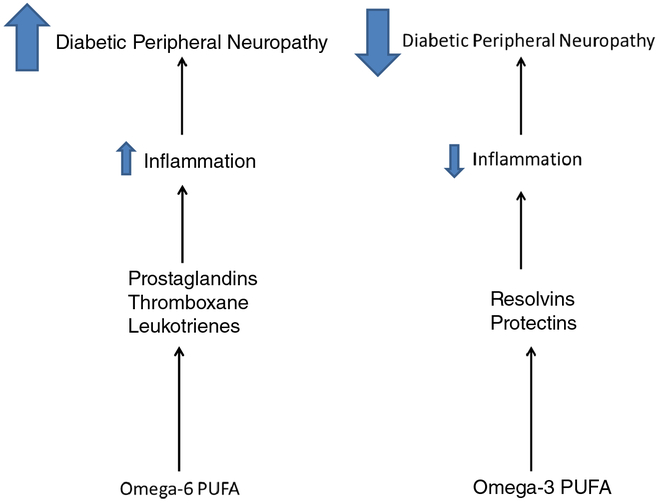
Diabetic peripheral neuropathy is a complex condition with multiple etiologies that can be influenced by lipids in a negative or positive manner (Fig. 2). Generally, saturated fatty acids and omega-6 polyunsaturated fatty acids negatively impact nerve function especially in obesity where abnormal adipose tissues and liver likely contribute to oxidative and inflammatory stress. The role of abnormal liver function in peripheral neuropathy is another interesting area that has been understudied. Recent studies in human subjects have linked non-alcoholic fatty liver disease with diabetes complications including chronic vascular complications and peripheral neuropathy [91–94]. Our rodent studies have provided similar evidence [22]. In our studies, we found that partially substituting oleic acid for saturated fatty acids did not provide any benefit to nerve function. This was not surprising since there was little change in the fatty acid profile of serum or tissues under this condition. However, partial replacement of saturated fatty acids derived from lard with omega-3 polyunsaturated fatty acids (derived from fish oil) in the diet of type 2 diabetic rats did improve vascular reactivity of epineurial arterioles of the sciatic nerve and neuropathy. We found that linoleic acid (omega-6 polyunsaturated fatty acid derived from safflower oil) was pro-inflammatory and aggravated vascular and neural function. However, enriching the diet of type 2 diabetic rats with δ linolenic acid (omega-6 polyunsaturated fatty acid derived from evening primrose oil) provided some benefit for vascular and neural function, although the greatest benefit was obtained when the diet was enriched with menhaden (fish) oil a source for the omega-3 eicosapentaenoic and docosahexaenoic acids. Enriching the diet with α-linolenic acid (omega-3 polyunsaturated fatty acid derived from flaxseed oil) also improved vascular and neural function, but it was not as efficacious as menhaden oil. The preclinical studies performed to date are insufficient and do not provide definitive proof that fish oil should be a recommended treatment for diabetic peripheral neuropathy. Future properly designed clinical studies are needed to determine whether fish oil can provide relief for peripheral neuropathy in human diabetic patients. Furthermore, fish oil treatment could only be one component of a successful treatment for diabetic peripheral neuropathy. Due to the multiple etiologies associated with diabetic neuropathy, the most effective treatment may be a combination of compounds that target these different mechanisms.
Fig. 2.
Effect of omega-6 and omega-3 PUFA on diabetic peripheral neuropathy
Footnotes
Conflict of Interest The author has no conflicts of interest to declare. The studies reported in this article from the author’s laboratory were supported by grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development [RX000889–06] and by the National Institute of Diabetes and Digestive and Kidney Diseases [DK107339–03] from NIH.
Human and Animal Rights and Informed Consent All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institution guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article includes the position statement from the ADA relating to peripheral neuropathy.
- 2.••.Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes. 2017;24:103–11. 10.1097/MED.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article describes how metabolic dysregulation as well as non-metabolic factors can contribute to diabetic neuropathy.
- 3.••.Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40:828–49. 10.1016/j.clinthera.2018.04.001. [DOI] [PubMed] [Google Scholar]; This review utilized a comprehensive literature search and provides background on the epidemiology; diagnosis, and the treatment of neuropathic pain in diabetic peripheral neuropathy.
- 4.Shin JY, Roh SG, Sharaf B, Lee NH. Risk of major limb amputation in diabetic foot ulcer and accompanying disease: a meta-analysis. J Plast Reconstr Aesthet Surg. 2017;70:1681–8. 10.1016/j.bjps.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Narres M, Kvitkina T, Claessen H, Droste S, Schuster B, Morbach S, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PLoS One. 2017;12:e0182081 10.1371/journal.pone.0182081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papanas N, Ziegler D. Emerging drugs for diabetic peripheral neuropathy and neuropathic pain. Expert Opin Emerg Drugs. 2016;21: 393–407. 10.1080/14728214.2016.1257605. [DOI] [PubMed] [Google Scholar]
- 7.Dy SM, Bennett WL, Sharma R, Zhang A, Waldfogel JM, Nesbit SA, et al. Preventing complications and treating symptoms of diabetic peripheral neuropathy. AHRQ Comparative Effectiveness Reviews Agency for Healthcare and Quality (US); 2017. March Report No:17-EHCOO5-EF. [PubMed] [Google Scholar]
- 8.Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: a update. Am Fam Physician. 2016;94: 227–34. [PubMed] [Google Scholar]
- 9.Dobrowsky RT. Targeting the diabetic chaperome to improve peripheral neuropathy. Curr Diab Rep. 2016;16:71 10.1007/s11892-016-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathy. Curr Diab Rep. 2016;16:29 10.1007/s11892-016-07275. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a recent review article describing inflammation as a target for diabetic neuropathy. The article includes how different pathogenic mechanisms thought to contribute to diabetic neuropathy increase inflammatory stress.
- 11.Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93:1021–7. 10.1139/cjpp2014-0353. [DOI] [PubMed] [Google Scholar]
- 12.••.Fernyhough P Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15:89 10.1007/s11892-015-0671-9. [DOI] [PubMed] [Google Scholar]; This was one of the earliest review article describing possible mechanisms responsible for mitochondrial dysfunction in diabetes.
- 13.Zhou J, Zhou S. Inflammation: therapeutic targets for diabetic neuropathy. Mol Neurobiol. 2014;49:536–46. 10.1007/s12035-013-8537-0. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxidative Med Cell Longev. 2013;2013: 168039–15. 10.1155/2013/168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varkonyi T, Putz Z, Keresztes K, Martos T, Lengyel C, Stirban A, et al. Current options and perspectives in the treatment of diabetic neuropathy. Curr Pharm Des. 2013;19:4981–5007. [DOI] [PubMed] [Google Scholar]
- 16.Stavniichuk R, Shevalye H, Lupachyk S, Obrosov A, Groves JT, Obrosova IG, et al. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014;30:669–78. 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupachyk S, Watcho P, Shevalye H, Vareniuk I, Obrosov A, Obrosova IG, et al. Na+/H+ exchanger 1 inhibition reverses manifestation of peripheral neuropathy in type 1 diabetic rats. Am J Physiol Endocrinol Metab. 2013;305:E396–404. 10.1152/ajpendo.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–52. 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, et al. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–55. 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 20.Malik RA. Why are there no good treatments for diabetic neuropathy? Lancet Diabetes Endocrinol. 2014;2:607–9. 10.1016/S2213-8587(14)70067-1. [DOI] [PubMed] [Google Scholar]
- 21.••.Malik RA. Wherefore art thou, O treatment for diabetic neuropathy? Int Rev Neurobiol. 2016;127:287–317. 10.1016/bs.irn.2016.03.008. [DOI] [PubMed] [Google Scholar]; This papers outlines some of the failures and issues associated with the lack of success in finding a treatment for diabetic neuropathy.
- 22.Holmes A, Coppey LJ, Davidson EP, Yorek MA. Rat models of diet-induced obesity and high fat/low dose streptozotocin type 2 diabetes: effect of reversal of high fat diet compared to treatment with enalapril or menhaden oil on glucose utilization and neuropathic endpoints. J Diabetes Res. 2015;307285:1–8. 10.1155/2015/307285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, Kardon RH, et al. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst. 2015;20:24–31. 10.1111/jns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson EP, Coppey LJ, Kardon RH, Yorek MA. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci. 2014;55:1222–30. 10.1167/iovs.13-13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson EP, Coppey LJ, Dake B, Yorek MA. Effect of treatment of Sprague-Dawley rats with AVE7688, enalapril, or candoxatril on diet-induced obesity. J Obes. 2011;686952:1–9. 10.1155/2011/686952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev. 2010;26:306–18. 10.1002/dmrr.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–608. 10.2337/db061176. [DOI] [PubMed] [Google Scholar]
- 28.Hinder LM, O’Brien PD, Hayes JM, Backus C, Solway AP, Sims-Robinson C, et al. Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis Model Mech. 2017;10: 717–25. 10.1242/dmm.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Tang D, Guan M, Xie C, Xue Y. Effect of high-fat diet on peripheral neuropathy in C57Bl/6 mice. Int J Endocrinol 2014:: 305205 Doi: 10.1155/2014/305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozay R, Uzar E, Aktas A, Uyar ME, Gurer B, Evliyaoglu O, et al. The role of oxidative stress and inflammatory response in high-fat diet induced peripheral neuropathy. J Chem Neuroanat. 2014;55: 51–7. 10.1016/j.jchemneu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 31.•.Cortez M, Singleton JR, Smith AG. Glucose intolerance, metabolic syndrome, and neuropathy. Handb Clin Neurol. 2014;126:109–22. 10.1016/B978-0-444-53480-4. [DOI] [PubMed] [Google Scholar]; This paper presents studies demonstrating that peripheral neuropathy develops in human subjects in the pre-diabetic phase.
- 32.Papanas N, Ziegler D. Prediabetic neuropathy: does it exist? Curr Diab Rep. 2012;12:376–83. 10.1007/s11892-0120278-3. [DOI] [PubMed] [Google Scholar]
- 33.Cho YR, Lim JH, Kim MY, Kim TW, Hong BY, Kim YS, et al. Therapeutic effects of fenofibrate on diabetic peripheral neuropathy by improving endothelial and neural survival in db/db mice. PLoS One. 2014;9:e83204 10.1371/journal.pone.0083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Othman A, Benghozi R, Alecu I, Wei Y, Niesor E, von Eckardstein A, et al. Fenofibrate lowers atypical sphingolipids in plasma of dyslipidemic patients: a novel approach for treating diabetic neuropathy? J Clin Lipidol. 2015;9:568–75. 10.1016/j.jacj.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Czupryniak L, Joshi SR, Gogtay JA, Lopez M. Effect of micronized fenofibrate on microvascular complications of type 2 diabetes: a systematic review. Expert Opin Pharmacother. 2016;17:1463–73. 10.1080/14656566.2016.1195811. [DOI] [PubMed] [Google Scholar]
- 36.Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Freemantle Diabetes Study. Diabetologia. 2008;51:562–6. 10.1007/s00125-007-0909-2. [DOI] [PubMed] [Google Scholar]
- 37.Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D’Emden MC, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373:1780–8. 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ACCORD Study Group, ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes. 2017;24:103–11. 10.1097/MED.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–50. [DOI] [PubMed] [Google Scholar]
- 41.Pitel S, Raccah D, Gerbi A, Pieroni G, Vague P, Coste TC. At low doses, a gamma-linolenic acid-lipoic acid conjugate is more effective than docosahexaenoic acid-enriched phospholipids in preventing neuropathy in diabetic rats. J Nutr. 2007;137:368–72. 10.1093/jn/137.2.368. [DOI] [PubMed] [Google Scholar]
- 42.Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003;68:151–62. [DOI] [PubMed] [Google Scholar]
- 43.Tao M, McDowell MA, Saydah SH, Eberhardt MS. Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2008;31:93–5. 10.2337/dc07-0931. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson DR, Robinson JP, Compton AM, Keen P. Essential fatty acid treatment: effects on nerve conduction, polyol pathway and axonal transport in streptozotocin diabetic rats. Diabetologia. 1989;32:655–9. [DOI] [PubMed] [Google Scholar]
- 45.Cameron NE, Cotter MA, Robertson S. Essential fatty acid diet supplementation. Effects on peripheral nerve and skeletal muscle function and capillarization in streptozotocin-induced diabetic rats. Diabetes. 1991;40:532–9. [DOI] [PubMed] [Google Scholar]
- 46.Burnard SL, McMurchie EJ, Leifert WR, Patten GS, Muggli R, Raederstorff D, et al. Cilazapril and dietary gamma-linolenic acid prevent the deficit in sciatic nerve conduction velocity in the streptozotocin diabetic rat. J Diabetes Complicat. 1998;12:65–73. [DOI] [PubMed] [Google Scholar]
- 47.Coste T, Pierlovisi M, Leonardi J, Dufayet D, Gerbi A, Lafont H, et al. Beneficial effects of gamma linolenic acid supplementation on nerve conduction velocity, Na+, K+ ATPase activity, and membrane fatty acid composition in sciatic nerve of diabetic rats. J Nutr Biochem. 1999;10:411–20. [DOI] [PubMed] [Google Scholar]
- 48.Cameron NE, Cotter MA, Horrobin DH, Trischler HJ. Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia. 1998;41:390–9. [DOI] [PubMed] [Google Scholar]
- 49.Hounsom L, Horrobin DF, Trischler H, Corder R, Tomlinson DR. A lipoic acid-gamma linolenic acid conjugate is effective against multiple indices of experimental diabetic neuropathy. Diabetologia. 1998;41:839–43. 10.1007/s001250050996. [DOI] [PubMed] [Google Scholar]
- 50.Shotton HR, Broadbent S, Lincoln J. Prevention and partial reversal of diabetes-induced changes in enteric nerves of the rat ileum by combined treatment with alpha-lipoic acid and evening primrose oil. Auton Neurosci. 2004;111:57–65. 10.1016/j.autneu.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Ford I, Cotter MA, Cameron NE, Greaves M. The effects of treatment with alpha-lipoic acid or evening primrose oil on vascular hemostatic and lipid risk factors, blood flow, and peripheral nerve conduction in the streptozotocin-diabetic rat. Metabolism. 2001;50: 868–75. 10.1053/meta.2001.24914. [DOI] [PubMed] [Google Scholar]
- 52.Jamal GA, Carmichael H. The effect of gamma-linolenic acid on human diabetic peripheral neuropathy: a double-blind placebo-controlled trial. Diabet Med. 1990;7:319–23. [DOI] [PubMed] [Google Scholar]
- 53.Keen H, Payan J, Allawi J, Walker J, Jamal GA, Weir AI, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The Gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8–15. [DOI] [PubMed] [Google Scholar]
- 54.Palacios-Palaez R, Lukiw W, Bazan N. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative diseases. Mol Neurobiol. 2010;41:367–74. 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- 55.Farooqui A n-3 fatty acid-derived lipid mediators in the brain: new weapons against oxidative stress and inflammation. Curr Med Chem. 2012;19:532–43. [DOI] [PubMed] [Google Scholar]
- 56.Coppey L, Holmes A, Davidson E, Yorek M. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab 2012: 950517 10.1155/2012/950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coppey L, Davidson E, Obrosov A, Yorek M. Enriching the diet with menhaden oil improves peripheral neuropathy in streptozotocin-induced type 1 diabetic rats. J Neurophysiol. 2015;113:701–8. 10.1152/jn.00718.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shevalye H, Yorek M, Coppey L, Holmes A, Harper MM, Kardon RH, et al. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol. 2015;114:199–208. 10.1152/jn.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davidson E, Holmes A, Coppey L, Yorek M. Effect of combination therapy consisting of enalapril, α-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. Eur J Pharmacol. 2015;765:258–67. 10.1016/j.ejphar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 60.••.Yorek M, Coppey L, Shevalye H, Obrosov A, Kardon R, Yorek M. Effect of treatment with salsalate, menhaden oil, combination of salsalate and menhaden oil, or resolvin D1 of C57Bl/6J type 1 diabetic mouse on neuropathic endpoints. J Nutr Metab 2016: 5905891 10.1016/j.ejphar.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first papers demonstrating that salsalate when in combination with fish oil increases resolvin production greater than fish oil alone.
- 61.Yorek MS, Obrosov A, Shevalye H, Coppey LJ, Kardon RH, Yorek MA. Early vs. late intervention of high fat/low dose streptozotocin treated C57Bl/6J mice with enalapril, α-lipoic acid, menhaden oil or their combination: Effect on diabetic neuropathy related endpoints. Neuropharmacology. 2017;116:122–31. 10.1016/j.neuropharm.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res. 1985;26:1015–35. [PubMed] [Google Scholar]
- 63.Yorek MA. Is fish oil a potential treatment for diabetic peripheral neuropathy? Curr Diabetes Rev. 2017; 10.2174/1573399813666170522155327. [DOI] [PubMed] [Google Scholar]
- 64.Roman-Pintos LM, Villegas-Rivera G, Rodriguez-Carrizalez AD, Miranda-Diaz AG, Cardona-Munoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res. 2016;3425617:1–16. 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14:473 10.1007/s11910-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerbi A, Maixent J, Ansaldi J, Pierlovisi M, Coste T, Pelissier JF, et al. Fish oil supplementation prevents diabetes-induec nerve conduction velocity and neuroanatomical changes in rats. J Nutr. 1999;129: 207–13. 10.1093/jn/129.1.207. [DOI] [PubMed] [Google Scholar]
- 67.Julu PO. Essential fatty acids prevent slowed nerve conduction in streptozotocin diabetic rats. J Diabet Complications. 1998;2:185–8. [DOI] [PubMed] [Google Scholar]
- 68.Heng L, Qi R, Yang R, Xu G. Docosahexaenoic acid inhibits mechanical allodynia and thermal hyperalgesia in diabetic rats by decreasing the excitability of DRG neurons. Exp Neurol. 2015;271: 291–300. 10.1016/j.expneurol.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 69.Li M, Wang Y, Cao R, Hou XH, Zhang L, Yang RH, et al. Dietary fish oil inhibits mechanical allodynia and thermal hyperalgesia in diabetic rats by blocking nuclear factor-κB-mediated inflammatory pathways. J Nutr Biochem. 2015;26:1147–55. https://doi.org/10.j.jnutbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Okuda Y, Mizutani M, Ogawa M, Sone H, Asano M, Asakura Y, et al. Long-term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. J Diabetes Complicat. 1996;10:280–7. [DOI] [PubMed] [Google Scholar]
- 71.••.Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology. 2017;88:2294–301. 10.1212/WNL.0000000000004033. [DOI] [PMC free article] [PubMed] [Google Scholar]; First paper to demonstrate that treating diabetic patients with a source of omega-3 polyunsaturated fatty acids promotes corneal nerve regeneration.
- 72.Pritchad N, Edwards K, Shahidi AM, Sampson GP, Russel AW, Malik RA, et al. Corneal markers of diabetic neuropathy. Ocul Surf. 2011;9:17–28. [DOI] [PubMed] [Google Scholar]
- 73.Travakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–7. 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malik RA. Early detection of nerve damage and repair in diabetic neuropathy. Nat Clin Pract Neurol. 2008;4:646–7. 10.1038/ncpneuro0938. [DOI] [PubMed] [Google Scholar]
- 75.Ariel A, Serhan C. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–83. [DOI] [PubMed] [Google Scholar]
- 76.Kohli P, Levy B. Resolvins and protectins: mediating solutions to inflammation. Brit J Pharmacol. 2009;158:960–71. 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cortina M, He J, Li N, Bazan N, Bazan H. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–10. 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon W, Bazan N. Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res. 2013;38:995–1005. 10.3109/02713683.2013.827211. [DOI] [PubMed] [Google Scholar]
- 79.Robson L, Dyall S, Sidloff D, Michael-Titus A. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurons throughout development and in aged animals. Neurobiol Aging. 2010;31:678–87. 10.1016/j.neurobiolaging.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 80.•.Obrosov A, Coppey LJ, Shevalye H, Yorek MA. Effect of fish oil vs. resolvin D1, E1, methyl esters of resolvins D1 or D2 on diabetic peripheral neuropathy. J Neurol Neurophysiol 2017:8: in press. Doi: 10.4172/2155-9562.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first paper to describe the effects of resolvin D1 and E1 on diabetic peripheral neuropathy.
- 81.••.Yorek MA. Is fish oil a potential treatment for diabetic peripheral neuropathy? Curr Diabetes Rev 2017. in press. Doi: 10.2174/1573399813666170522155327. [DOI] [PubMed] [Google Scholar]; A recent review article describing the potential benefits of fish oil on diabetic neuropathy.
- 82.Coppey LJ, Shevalye H, Obrosov A, Davidson EP, Yorek MA. Determination of peripheral neuropathy in high-fat diet fed lowdose streptozotocin-treated female C57Bl/6J mice and Sprague-Dawley rats. J Diabetes Investig. 2018; 10.1111/jdi.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Shetty S, Zhang P, Gao R, Hu Y, Wang S, et al. Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Tox Appl Pharma. 2014;277:118–23. 10.1016/j.taap.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Dam AD, Nahon KJ, Kooijman S, van den Berg SM, Kanhai AA, Kikuchi T, et al. Salsalate activates brown adipose tissue in mice. Diabetes. 2015;64:1544–54. 10.2337/db141125. [DOI] [PubMed] [Google Scholar]
- 85.Chai W, Liu J, Fowler D, Barrett E, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care. 2011;34:1634–8. 10.2337/dc10-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ariel D, Kim SH, Liu A, Abbasi F, Lamendola CA, Grove K, et al. Salsalate-induced changes in lipid, lipoprotein, and apoprotein concentrations in overweight or obese, insulin-resistant, nondiabetic individuals. J Clinical Lipidol. 2015;9:658–63. 10.1016/j.jacl.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldfine A, Fonseca V, Jablonski K, Pyle L, Staten M, Shoelson S. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. 10.7326/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, et al. Salicylate (salsalate) in patients with type 2 diabetes. Ann Intern Med. 2013;159:1–12. 10.7326/0003-4819159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA. Effect of dietary content of menhaden oil with or without salsalate on neuropathic endpoints in high fat fed/low dose streptozotocin treated Sprague-Dawley rats. J Diabetes Res. 2018;2969127 10.1155/2018/2967127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantovani A, Rigolon R, Mingolla L, Pichiri I, Cavalieri V, Salvotelli L, et al. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J Diabetes Complicat. 2017;31:1021–6. 10.1016/j.jdiacomp.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 92.Williams KH, Burns K, Constantino M, Shackel NA, Prakoso E, Wong J, et al. An association of large-fibre peripheral nerve dysfunction with non-invasive measures of liver fibrosis secondary to non-alcoholic fatty liver disease in diabetes. J Diabetes Complicat. 2015;29:1240–7. 10.1016/j.jdiacomp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev. 2018;14:99–114. 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 94.Lv WS, Sun RX, Gao YY, Wen JP, Pan RF, Li L, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol. 2013;19:3134–42. 10.3748/wjg.v19.i20.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]