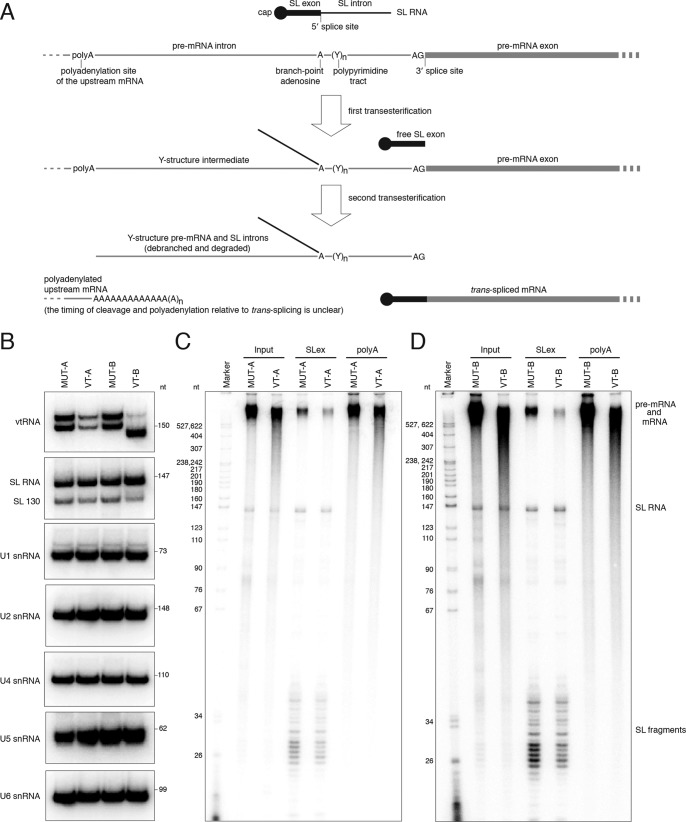
Figure 7.
vtRNA in T. brucei is required for the production of trans-spliced mRNA. A, diagram of the mechanism of coupled trans-splicing and polyadenylation in T. brucei. Note that the timing of splicing and upstream mRNA polyadenylation is currently unknown. Efficiency of processing events upstream or downstream of any mRNA could result in species that are either not spliced or not polyadenylated. B, Northern blot analysis of the indicated RNAs in permeabilized BF cells treated with the vtRNA antisense oligodeoxynucleotide VT-A or VT-B or their mutant versions, MUT-A or MUT-B, as controls. C, analysis of newly synthesized, 32P-labeled RNA in permeabilized BF cells treated with MUT-A or VT-A oligo. Input is the total cellular RNA; SLex samples represent the RNA enriched on streptavidin beads coupled to biotinylated antisense oligo against the SL exon sequence; poly(A) is the RNA enriched on beads with biotinylated (dT)39 oligonucleotide; D, as in C, but for cells treated with the MUT-B or VT-B oligo. Marker is a 32P-labeled pBR322 DNA MspI digest. Indicated are the positions of the SL RNA and SL RNA fragments. As the conditions for the assay were optimized for pol II transcription, the majority of the high-molecular-weight signal represents pre-mRNA and mRNA.