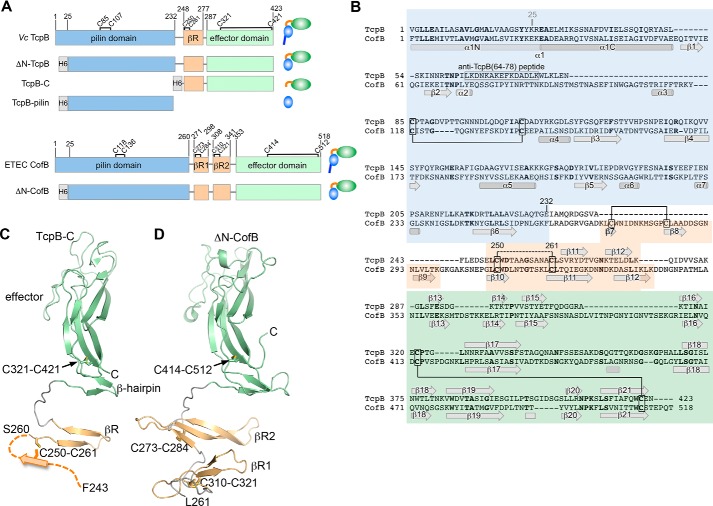
Figure 1.
Structure of the C-terminal half of V. cholerae TcpB and comparison with ETEC CofB. A, domain organization of TcpB and CofB and their recombinantly expressed forms. Domain boundaries and cysteines are indicated. B, amino acid sequences of TcpB and CofB aligned based on their structures and colored as in A. Identical residues are shown in boldface text. TcpB and CofB are 20% identical in their C-terminal halves (β-repeat and effector domain) and 16% identical overall. Disulfide bonds seen in the crystal structures are indicated with solid lines. The disulfide bond predicted between TcpB Cys-250 and Cys-261 is shown with a dashed line. The peptide used to generate anti-TcpB(64–78) antibodies is indicated. C, crystal structure of TcpB-C (residues 243–423) shown in ribbon representation, colored and labeled as in A and B. Residues 243–259 are not resolved in the crystal structure and are illustrated as the first strand of the three-stranded βR based on their alignment with βR2 of CofB. D, crystal structure of ΔN-CofB (22). Only the C-terminal half of ΔN-CofB (residues 261–518) is shown, omitting the pilin domain.