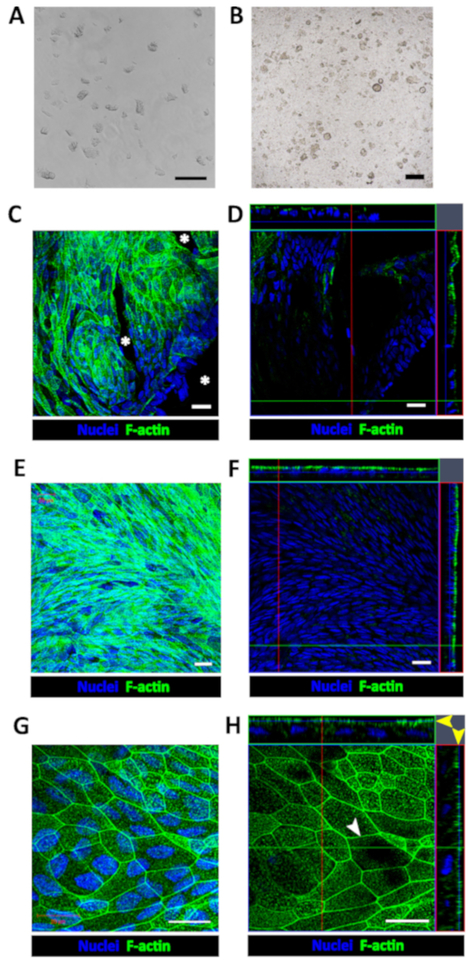
Figure 1: Establishment of human enteroid/colonoid monolayers.
(A) Example of colonoid fragments after the dissolution of BMM and trituration. (B) Example of insert immediately after plating colonoid fragments. Scale bar (A-B) = 200 μm. Representative (C) maximum intensity projection and (D) confocal optical Z-section with the corresponding orthogonal projections show that colonoid fragments seeded onto human collagen IV-coated filters form multiple monolayer islands 2–4 days post-seeding. Cell-free areas (asterisk) are identifiable by the absence of both nuclear (Hoechst 33342, blue) and apical F-actin (phalloidin, green) staining in both C and D. Representative (E) maximum intensity projection and (F) confocal optical section with the corresponding orthogonal projections show a confluent colonoid monolayer with continuous apical surface detected by F-actin immunostaining approximately 1-week post-seeding. (G) High magnification of a representative maximum intensity projection and (H) confocal optical section with the corresponding orthogonal projections show that cells in confluent colonoid monolayers form the F-actin perijunctional rings (white arrowhead) and an immature apical brush border (yellow arrow heads). Scale bar (C–H) = 20 μm.