Figure 3.
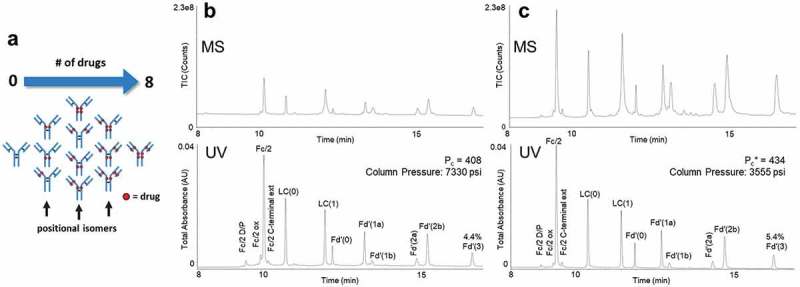
(a) Representation of the different possible drug load distributions of cysteine-conjugated ADCs.18 Subunits from a cysteine-linked auristatin-conjugated antibody as separated with (b) a C4-bonded organosilica 300 Å fully porous stationary phase, 0.6 mL/min flow rate, 80°C temperature, 0.1% TFA-modified mobile phases, and 90:10 acetonitrile/IPA eluent versus (c) a method consisting of a phenyl bonded 2.7 µm superficially porous 450 Å stationary phase, 0.6 mL/min flow rate, 70°C temperature, and 0.15% DFA-modified mobile phases.
