Figure 1.
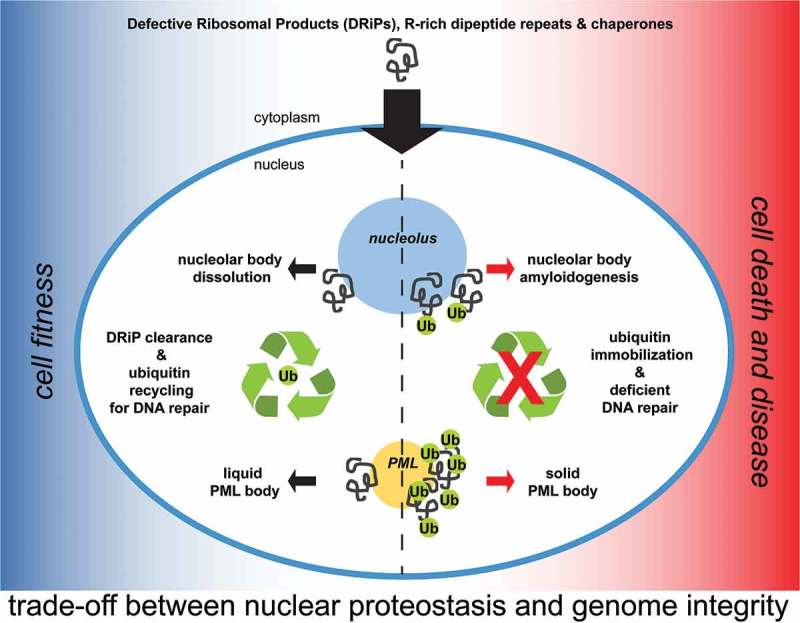
Role of nucleoli and PML-bodies in nuclear proteostasis. A fraction of low molecular weight DRiPs escapes the cytoplasmic protein quality control system and enters the nucleus. DRiPs are transiently stored in nucleolar subcompartments (NoABs) for later disposal by chaperones and proteasomes. Upon proteotoxic stress conditions, such as heat shock, and proteasome inhibition, a fraction of DRiPs is transiently compartmentalized at PML-bodies, which act as stress-responsive overflow compartments. Along with DRiPs, PML-bodies recruit ubiquitin, chaperones and proteasomes to promote efficient DRiP degradation. Failure to clear DRiPs from NoABs and PML-bodies leads to their solidification and immobilizes ubiquitin and proteasomes. This, in turn, depletes the pool of free ubiquitin to the detriment of the ubiquitin-dependent labeling of DNA damaged sites.
