Figure 1.
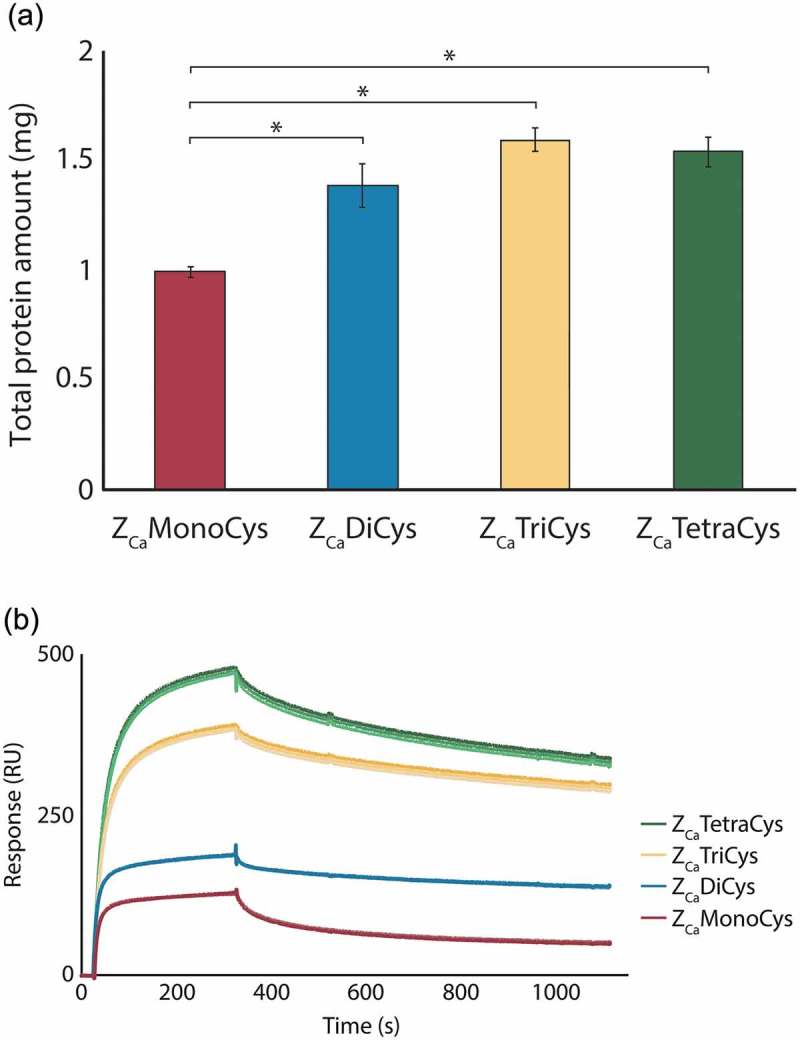
Monomeric and multimeric ZCa variants with C-terminal cysteines analyzed as ligands in a column purification setup and by SPR (a) The average amount (±SD) of human polyclonal IgG eluted with EDTA at pH 5.5 from columns coupled with the mono- (red), di- (blue), tri- (yellow) and tetrameric (green) ZCa, based on triplicate experiments. The multimeric variants of ZCa capture significantly more protein as compared to the monomer (*P < .001). (b) Sensorgrams from triplicate injections of 500 nM IgG flown over surfaces with equimolar amounts of each ZCa variant immobilized show that larger multimers exhibit improved binding performance.
