Abstract
Introduction
We aimed to examine the contribution of subjective cognitive decline (SCD) to reduce the number of β-amyloid (Aβ) positron emission tomography scans required for recruiting Aβ+ clinically normal individuals in clinical trials.
Methods
Three independent cohorts (890 clinically normal: 72 yrs ± 6.7; Female: 43.4%; SCD+: 24%; apolipoprotein E [APOE] ε4+: 28.5%; Aβ+: 32%) were used. SCD was dichotomized from one question. Using logistic regression, we classified Aβ+ using the SCD dichotomy, APOEε4, sex, and age.
Results
SCD increased odds of Aβ+ by 1.58 relative to non-SCD. Female APOEε4 carriers with SCD exhibited higher odds of Aβ+ (OR = 3.34), whereas male carriers with SCD showed a weaker, opposing effect (OR = 0.37). SCD endorsement reduces the number of Aβ positron emission tomography scans to recruit Aβ+ individuals by 13% and by 9% if APOEε4 status is known.
Conclusion
SCD helps to classify those with high Aβ, even beyond the substantial effect of APOE genotype. Collecting SCD is a feasible method for targeting recruitment for those likely on the AD trajectory.
Keywords: Subjective cognitive decline, Amyloid, APOEε4, Alzheimer's disease
Highlights
-
•
SCD can significantly identify high Aβ beyond basic demographics and APOEε4.
-
•
Female APOEε4 carriers with SCD have greater odds for high Aβ relative to males.
-
•
SCD+ reduces the number of Aβ-PET scans required by approximately 13%.
1. Introduction
As the field increasingly moves toward earlier intervention for Alzheimer's disease (AD), there is a growing need for reliable and cost-effective ways of screening for individuals who will benefit most from timely prevention. Current prevention efforts are predominantly focused on interrupting the pathological β-amyloid (Aβ) cascade in its earliest stages [1]. As such, recruitment is predicated on identifying individuals with biomarker evidence of abnormal Aβ using positron emission tomography (PET) neuroimaging [2]. Aβ-PET imaging is expensive and invasive, and so to reduce cost and patient burden, prevention trials may harness easily accessible demographic factors to prescreen individuals before neuroimaging. Beyond gathering information about apolipoprotein E ε4 (APOEε4) status, which is closely associated with risk for abnormal Aβ [3], [4], and age [5], [6], evidence also supports the inclusion of measures of subjective cognitive decline (SCD) for prescreening [7], [8], [9].
As a quick and inexpensive marker, SCD has been repeatedly associated with Aβ burden in clinically normal older adults [3], [7], [8], [10], [11], [12], [13], [14], [15], [16]. When examining the predictive utility of SCD to identify high Aβ, Mielke et al. [5] reported that SCD reduced the number of individuals needed to screen for high Aβ burden by approximately 37% for clinically normal individuals between 70 and 79 years. Furthermore, a study from the Australian Imaging, Biomarker and Lifestyle (AIBL) study of aging reported that SCD increased the odds of high Aβ by 1.90 in clinically normal individuals, raising to an odds ratio (OR) of 4.58 in APOEε4 carriers [17]. A current gap in the literature, however, is the reporting of risk estimates for high Aβ using SCD across multiple independent cohorts. This is a salient issue for SCD, which is a multifaceted construct [18] that does not currently possess a standardized form of measurement [19]. As such, the aim of this study was to examine the generalizability and consistency of SCD to identify high Aβ across three well-characterized cohorts, and in the context of demographic and genetic factors.
Here, we examined the utility of SCD (as measured with a single question with a binary response) to identify high Aβ in isolation and in combination with moderative effects of APOEε4 status, age, and sex across 890 clinically normal older adults from three independent cohorts. We hypothesized that SCD would exert an independent effect on the identification of those with high Aβ and that combinatorial relationships between SCD and demographic factors would significantly reduce the numbers to screen for high Aβ.
2. Methods
2.1. Participants
Cohort-specific inclusion criteria for recruitment have been published previously in the following studies: Harvard Aging Brain Study (HABS), Alzheimer's disease Neuroimaging Initiative (ADNI), and AIBL [20], [21], [22]. For this cross-sectional study, data from each cohort were based on an individual's first Aβ positron emission tomography (PET) scan. In the present study, participants were required to be clinically normal (Global Clinical Dementia Rating [CDR] score = 0), with ADNI's SCD group included, given that these participants attained a Clinical Dementia Rating score of 0. For analysis, 890 participants (ADNI, n = 297; AIBL, n = 284; and HABS, n = 309) were included. We conducted the procedures for this study under the ethical guidelines stipulated by the Partners Human Research Committee, which is the Institutional Review Board for the Massachusetts General Hospital and Brigham and Women's Hospital.
2.2. Subjective cognitive decline
We examined SCD using three binary outcome questions—from ADNI, we used the Everyday Cognition battery [23] memory question: “Are you concerned that you have a memory or other thinking problem?” [24]; from AIBL: “Do you have difficulties with your memory?” [21]; and from HABS, we used the first question from the Structured Telephone Interview for Dementia Assessment [25]: “Have you recently experienced any change in your ability to remember things?” [26]. These questions were used to identify SCD, with endorsement (“yes”) signifying those with a subjective observation of poor memory.
2.3. Aβ positron emission tomography
ADNI uses the 18F-AV45 (florbetapir or FBP) Aβ-PET tracer, whereas AIBL and HABS use the 11C-Pittsburgh compound-B (PiB) Aβ-PET tracer. The PET acquisition parameters and processing pipelines for each study have been published previously [3], [22], [27]. AIBL and HABS used cerebellar gray matter as the reference region, whereas ADNI used the whole cerebellum as the reference region. While ADNI and AIBL used standardized uptake value ratios, HABS used distribution value ratio. We used dichotomous Aβ status using previously published cutoff values [3], [27], [28]: AIBL > 1.40 standardized uptake value ratios; ADNI > 1.11 standardized uptake value ratios; and HABS > 1.185 distribution value ratio.
2.4. Statistical analysis
Analyses were performed using R, version 3.5.1, using the glm, pscl, and epiDisplay packages. To examine the ability of SCD to classify high/low Aβ burden in clinically normal older adults, we ran a series of generalized linear mixed models including age, sex, APOEε4, and education as fixed effect covariates and modeling cohort as a random effect. Our first model included demographics, followed by a second model which included SCD as a predictor of interest. The next model examined demographics and APOEε4 status as a predictor of interest. Our examination of APOEε4 status as the primary contrast against the effect of SCD on Aβ status was motivated by the fact that APOEε4 represents one of the strongest risk factors for high Aβ [4], [29]. To examine the added effect of SCD beyond knowing APOEε4 status, we examined the main effects of SCD and APOEε4 within the same model. To examine interactive effects, we examined the combined effect of SCD and APOEε4 status on Aβ status. We also examined two additional models that included three-way interactions between (a) sex, SCD, and APOEε4 status and (b) age, SCD, and APOEε4 status on Aβ status. We investigated the fit of these models with area under the curve (AUC) calculations. Although we proceeded with three-way interactions as we had >20 individuals in each cell for both Aβ+ and Aβ− individuals (see Supplementary Table A), we interpreted results within the context of reduced power. In addition, we calculated the numerical advantage to including SCD as a parameter when attempting to recruit 1000 Aβ+ clinically normal individuals from the community. Here, we used the predictive models to calculate the percentage reduction in numbers needed to recruit 1000 Aβ+ individuals by predicting the ŷ from a reference version of the model (that is, all variables at “zero”) and comparing it against the target version of the model (that is, with the variable of interest now set to be the indicator).
3. Results
3.1. Demographics
Clinically normal older adults who had high Aβ burden were older and had greater proportion of APOEε4 and endorsement of SCD (see Table 1) across all cohorts. There were no differences in years of education or proportion of females across all cohorts.
Table 1.
Demographic differences by amyloid status and cohort
| Aβ− (n = 607) | Aβ+ (n = 283) | P | |
|---|---|---|---|
| Age | 71.6 (6.9) | 75.0 (6.3) | <.001 |
| Education (yrs) | 15.1 (3.2) | 14.9 (3.4) | .31 |
| Sex (F%) | 337 (56) | 165 (58) | .48 |
| APOE (ε4+ %) | 116 (20) | 131 (48) | <.001 |
| SCD (yes %) | 272 (49) | 157 (56) | .004 |
| ADNI (n = 201) | AIBL (n = 182) | HABS (n = 224) | ADNI (n = 96) | AIBL (n = 102) | HABS (n = 85) | ||
|---|---|---|---|---|---|---|---|
| Age | 72.9 (6.1) | 70.2* (6.2) | 71.7 (7.7) | 75.9 (6.2)* | 74.0 (6.4)* | 75.3 (6.3)* | <.001 |
| Education (yrs) | 16.8 (2.4) | 12.6 (2.6)* | 16.1 (2.8) | 16.1 (2.8) | 12.5 (3.0)* | 16.2 (2.9) | |
| Sex (F%) | 95 (47) | 109 (60)* | 109 (49) | 63 (66)* | 51 (50) | 51 (60) | .02 |
| APOE (ε4+ %) | 41 (20) | 37 (21) | 38 (18) | 42 (44)* | 41 (43)* | 48 (59)* | <.001 |
| SCD (yes %) | 87 (43) | 126 (69)* | 59 (26) | 45 (47) | 72 (71)* | 40 (47) | <.001 |
NOTE. * = significantly different from other groups.
Abbreviations: ADNI, Alzheimer's disease Neuroimaging Initiative; AIBL, Australian Imaging, Biomarker and Lifestyle; HABS, Harvard Aging Brain Study; APOE, apolipoprotein E.
3.2. Classifying Aβ status using SCD and APOEε4 status
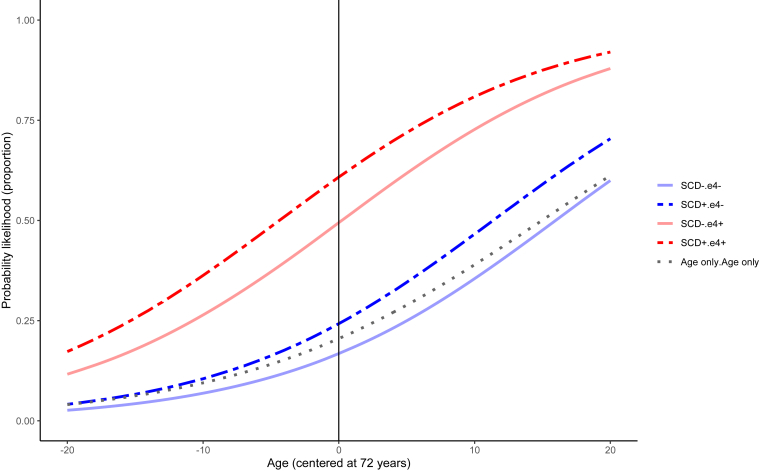
In the most basic model examining demographics alone, the AUC was 66%. As education level did not add significant explanatory variance to classifying Aβ+, we removed it from subsequent models. When including SCD in the model, we found it elevated the odds of being Aβ+ by 1.58 (P = .006, 95% confidence interval [CI]: 1.21-2.07) relative to non-SCD, with an AUC of 66.2% (see Table 2 for model estimates). This model was significantly better fitting than that including demographics alone (χ2 = 7.35, P < .001). In a model that included APOEε4 status and demographics, being an APOEε4 carrier increased the odds of being Aβ+ by 4.94 (P < .001, 95% CI: 2.08-11.72) and the AUC was 74.8% model fitting against demographics alone (χ2 = 88.6, P < .001). Including the main effects of both SCD and APOEε4 status, SCD maintained its significance (OR = 1.52, 95% CI: 1.17-1.98, P = .01), with the AUC at 74.7% (see Fig. 1). This model fit better than that including only APOEε4 status and demographics (χ2 = 6.03, P = .01). We found no significant two-way interaction between SCD and APOEε4 status to identify Aβ+ (OR = 0.95, 95% CI: 0.69-1.32, P = .89; AUC = 74.8%) (see Fig. 1).
Table 2.
Odds ratios for variables of interest in examined models
| Odds ratio | 95% CI lower | 95% CI upper | P-value | |
|---|---|---|---|---|
| Aβ status ∼ SCD + covariates: AUC = 66.2 | ||||
| SCD+ | 1.58 | 1.21 | 2.07 | 0.006 |
| Female | 1.32 | 0.97 | 1.79 | 0.08 |
| Age | 1.09 | 1.06 | 1.11 | <0.001 |
| Aβ status ∼ APOE + covariates: AUC = 74.8 | ||||
| APOEε4+ | 4.94 | 2.08 | 11.72 | <0.001 |
| Female | 1.29 | 1.05 | 1.60 | 0.11 |
| Age | 1.10 | 1.09 | 1.12 | <0.001 |
| Aβ status ∼ SCD + APOE + covariates: AUC = 74.7 | ||||
| SCD+ | 1.52 | 1.17 | 1.98 | 0.01 |
| APOEε4+ | 4.87 | 3.47 | 6.89 | <0.001 |
| Female | 1.24 | 0.90 | 1.72 | 0.20 |
| Age | 1.11 | 1.08 | 1.14 | <0.001 |
| Aβ status ∼ SCD*APOE + covariates: AUC = 74.8 | ||||
| SCD+ | 1.56 | 1.05 | 2.34 | 0.03 |
| APOEε4+ | 5.04 | 3.11 | 8.24 | <0.001 |
| APOEε4+:SCD+ | 0.95 | 0.69 | 1.32 | 0.89 |
| Aβ status ∼ SCD*APOE*Age + covariates: AUC = 74.8 | ||||
| SCD+ | 1.66 | 1.08 | 2.58 | 0.02 |
| APOEε4+ | 5.17 | 3.15 | 8.61 | <0.001 |
| Age | 1.12 | 1.07 | 1.17 | <0.001 |
| APOEε4+:Age:SCD+ | 1.06 | 1.00 | 1.13 | 0.25 |
| Aβ status ∼ SCD*APOE*Sex + covariates: AUC = 75.7 | ||||
| SCD+ | 1.51 | 0.83 | 2.75 | 0.18 |
| APOEε4+ | 10.74 | 5.18 | 22.94 | <0.001 |
| Female | 1.38 | 0.78 | 2.45 | 0.28 |
| APOEε4+:Female:SCD+ | 6.60 | 1.63 | 25.32 | 0.007 |
| Aβ status ∼ SCD + covariates in female APOEε4 carriers (n = 150) | ||||
| SCD+ | 3.34 | 1.65 | 7.00 | 0.001 |
| Age | 1.09 | 1.04 | 1.16 | 0.001 |
| Aβ status ∼ SCD + covariates in male APOEε4 carriers (n = 97) | ||||
| SCD+ | 0.37 | 0.13 | 1.00 | 0.05 |
| Age | 1.21 | 1.12 | 1.34 | <0.001 |
Abbreviations: APOE, apolipoprotein E; AUC, area under the curve; SCD, subjective cognitive decline.
Fig. 1.
Probability of having high Aβ according to SCD endorsement and APOEε4 carrier status. Abbreviations: APOE, apolipoprotein E; SCD, subjective cognitive decline.
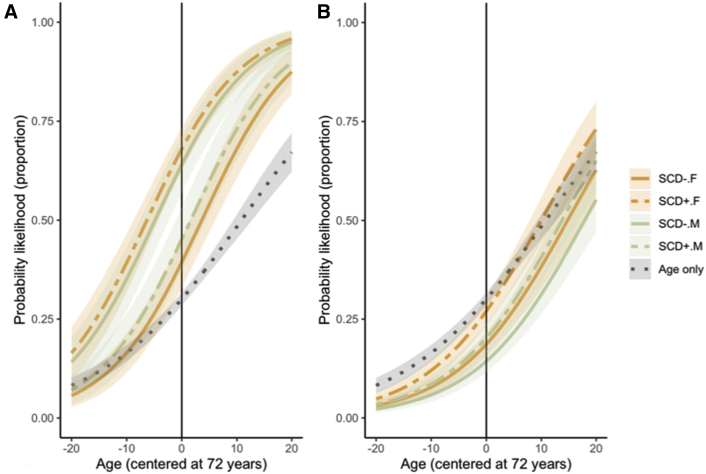
For the three-way interactions, there were none between age, SCD, and APOEε4 status to identify Aβ+ (OR = 1.06, 95% CI: 1.00-1.13, P = .25; AUC = 74.8%). A three-way interaction was found to exist, however, between sex, SCD, and APOEε4 status (OR = 6.60, 95% CI: 1.63-25.32, P = .007; AUC = 75.7%). This model fit better than that of a main-effect-only model (χ2 = 13.05, P = .01). Stratified by APOEε4 carriers, females had significantly greater odds of being Aβ+ if they endorsed SCD (OR = 3.34, 95% CI: 1.65-7.00, P = .001) (see Fig. 2). Unexpectedly, male APOEε4 carriers exhibited trend-level lower odds of being Aβ+ if they endorsed SCD (OR = 0.37, 95% CI: 0.13-1.00, P = .05). A visual inspection of the three-way estimate across cohorts also suggests that they were largely aligned across cohorts (see Supplementary Figure A). When examining the stratifications within each study, the ORs for female ε4 carriers were relatively similar across all studies (ADNI: 4.25 [1.24-16.69]; AIBL: 3.98 [0.86-22.31]; and HABS: 2.38 [0.78-7.53]), with ADNI providing the strongest effect. Although the direction of the OR estimates was similar for male carriers, the estimate was far lower in ADNI in comparison with AIBL and HABS samples (ADNI: 0.02 [0.00-0.51]; AIBL: 0.30 [0.04-1.48]; and HABS: 0.86 [0.14-5.71]).
Fig. 2.
Probability of having high Aβ according to SCD endorsement and sex in (A) APOEε4 carriers and (B) noncarriers. Abbreviations: APOE, apolipoprotein E; SCD, subjective cognitive decline.
3.3. Estimated scans needed to identify 1000 Aβ+ in clinically normal older adults
Knowing SCD alone beyond the average demographics of the cohorts (age = 72 years, sex = 56% female treated as numeric, cohort treated as numeric) reduced the numbers needed to scan by 13% [95% CI: 11-16%] (from 3276 to 2867 scans, saving 409 scans [95% CI: 393-485 scans saved]). Knowing both APOEε4 status and SCD translated to a reduction in the number of scans by 49% [95% CI: 49-51%] over and above basic demographics (3276 scans to 1655; saving 1621 scans [95% CI: 1531-1832]). In addition to knowing APOEε4 status, SCD itself only contributed to a reduction in the number of scans by approximately 9% [95% CI: 8-9%] in comparison with a model including APOEε4 and demographics saving 154 scans [95% CI: 144-165]). Across the cohorts, there was some level of variability in the estimation of number of scans saved here; within AIBL, ADNI, and HABS, the number of scans estimated to be saved by knowing SCD above APOEε4 and demographics was 4%, 2%, and 13%, respectively.
It is important to note here, however, that simply estimating the numbers needed to be scanned does not take into account the costs of genotyping and other issues that may be pertinent to decision-making with recruitment. The intent of these estimations is primarily to highlight the utility of including an SCD measure to increase the efficiency of preclinical recruitment (with the inclusion of CIs).
4. Discussion
The field is currently focused on better, and more efficiently, identifying the most at-risk, yet clinically healthy, individuals who are “trial ready.” In the present study, we aimed to quantify the contribution of a relatively simple and cheap approach to classifying abnormal Aβ, assessing SCD using a single item, across three independent observational cohorts. We found that SCD significantly predicted high Aβ, and this effect remained after accounting for APOEε4 status, although it was relatively small in comparison with the APOE effect. Including SCD in the identification of those with high Aβ reduced the numbers needed to screen by 13% beyond solely knowing basic demographics, such as age and sex, and by 9% if APOEε4 status was known. If one assumes the cost of an Aβ PET scan is ∼US $3500, recruitment strategies based on inclusion of SCD, over and above basic demographics, could boost economic efficiency by approximately $1,431,500 [95% CI $1,375,500-$1,697,500], estimated from the 409 scans saved. This may not be considered a large margin of reduction; however, the inclusion of an additional one question is both low in cost and time expenditure and would be of net benefit for screening procedures of those with high Aβ. An important consideration, however, is the extent to which the endorsement of SCD differed in its predictive utility across the cohorts, suggesting that some items may be more sensitive to identifying high Aβ. The issue of other idiosyncratic differences between the cohorts, however, cannot be discounted to explain this variation.
Taken together, the literature supports our findings that SCD, age, and APOEε4 can inform the likelihood of high Aβ burden in community samples of clinically normal individuals. Elevated SCD, as a continuous measure, is associated with continuous measures of Aβ [7], [8], [13], [30]. Our estimates of high Aβ frequency in APOEε4 carriers and noncarriers also align with those from an unrelated cohort [29], and meta-analyses incorporating some of the cohorts used in the present study [4], implying that our sample is representative. Similarly, we replicated findings from other independent cohorts of the relationship between higher Aβ and increased age [5], [6]. Meta-analyses do not support a relationship between sex and high Aβ [4], [31], [32], although one observational study reported a female bias [33]. We extend these findings by reporting the magnitude of predictive utility that SCD measurement can provide about the likelihood of Aβ positivity in combination with predominating factors (e.g., age, APOE).
Our estimate of SCD to identify high Aβ was lower than that presented by Mielke et al. (13% vs. 34% [5]). One possibility for this discrepancy is the use of only a single binary SCD question in the present study, which may result in reduced sensitivity. In addition, the cohorts we examined displayed a trend-level protective effect of SCD in males, which may have obscured the impact of SCD in females. We also did not find interactions between SCD and age to predict high Aβ, as per previous findings [5], [17], suggesting that although age exerts a strong main effect, the effect of SCD is not more salient in certain age groups.
The highest explanatory power for identifying high Aβ resulted from combinations of factors. We found the highest odds in female APOEε4 carriers with SCD, with our findings showing a paradoxical protective effect in male carriers with SCD. SCD and sex have not traditionally been associated with one another in relation to Aβ burden; however, some meta-analyses have suggested elevated SCD overall in females relative to males [34]. Medical-seeking behaviors are reported more strongly in females relative to males (in the present study, 51% of females endorsed SCD relative to 45% of males), supporting the notion that SCD may be more common in females. It remains unclear, however, why SCD in females may be more sensitive to high Aβ. One possible rationale is a higher awareness for detecting cognitive changes [35]. Alternatively, a single binary outcome measure could be perceived differently by each sex, although this remains to be explored further.
As these cohorts are community based, it is unclear what the utility of SCD is for identifying Aβ in clinical settings. Because memory-clinic groups with SCD are at greater risk for clinical progression to AD dementia than the general population [36] (with SCD potentially more informative for clinical progression [37]), it is entirely possible that our estimates may be underestimating the risk for high Aβ in this type of population. Alternatively, SCD endorsement likely underlies the presenting symptom to a memory clinic, thus reducing the utility of a single binary outcome of SCD in this type of population. An additional consideration is that a variety of methods of SCD measurement exist, from single binary outcome measures to longer Likert-scale questionnaires [19]; we did not examine the sensitivity of different SCD measures to identify high Aβ. Our aim was to examine the generalizability of SCD based on items that were most comparable across the three cohorts while still retaining face validity. It is important to note, however, that this approach to testing SCD may not be the most sensitive to detecting abnormal Aβ in the community. Generalized SCD questions may promote reflection on a compilation of experiences related to SCD [38] and thus result in a heterogeneous association with AD pathophysiology. Arguably, however, all forms of SCD measurement are more feasible and cost-effective than attaining APOE genotype and, as such, should be considered for implementation as a recruitment and screening tool in the first instance [9].
Strengths of the present study include the consolidation of three independent cohorts to form a large sample of community-based older adults, as well as considering cohort variance. Some limitations exist in this study. First, we did not have the same measure of SCD across all three cohorts, which could lead to some level of measurement error and subsequent misestimation of risk. We did find our estimates of interest were largely aligned across cohorts, thus supporting the notion of generalizability of the SCD construct to identify high Aβ. Some heterogeneity did exist in our models, however; in particular, ADNI seemed to exhibit the strongest effect of SCD on high Aβ. We attempted to account for this by including cohort as a random effect; however, this limitation should be acknowledged. There are also previously acknowledged issues with these convenience-sample cohorts that reduce generalizability, such as high education, good physical health, low racial diversity, and in the case of the AIBL study, enrichment for APOEε4 status [21]. An additional consideration is that each of these binary outcome questions asks about a slightly different component of SCD: one asks about concern, another about difficulties, whereas the last asks about experiencing change. All of these questions tap into different facets of SCD [19], and so it remains unclear which elements may have the greatest sensitivity to high Aβ or whether they are interchangeable. We did find a difference in the frequency of SCD endorsement across the cohorts, suggesting that they may not be entirely interchangeable. Regardless, asking a single question about SCD is time-efficient, cost-effective, and does not require training or clinical acumen to acquire, making this form of measurement an ideal addition to gathering simple demographic information in a first level of screening for recruitment [9].
In this large, combined sample, we found a single, binary SCD question independently identified high Aβ in clinically normal older adults. The predictive utility remained even after including APOE genotype in the model. Future aims will be to examine the predictive utility of SCD to identify groups according to the A/T/N model [39]. In addition, it will be necessary to examine the impact of screening questions for SCD that hone on different features that reflect elements in the new NIA-AA criteria [39] and/or SCD-plus criteria from the SCD-Initiative [18].
Research in Context.
-
1.
Systematic review: The authors reviewed literature in PubMed and Google Scholar. Some literature exists on the predictive utility of subjective cognitive decline (SCD) to identify high Aβ for Alzheimer's disease prevention trials. Studies have yet to determine the generalizability of these predictive estimates across independent cohorts.
-
2.
Interpretation: Our findings underscore the importance of including a measure of SCD in prescreening recruitment methods for high Aβ, even if it is a single binary question (as reported across three cohorts in the present study).
-
3.
Future directions: Because identifying high Aβ using Aβ positron emission tomography is so expensive, but currently represents the gold standard for recruitment into prevention trials, our results highlight the utility of a cheap and time-efficient measure of SCD to identify clinically normal older adults with high Aβ. Further work should explore the use of different items of SCD to classify Aβ.
Acknowledgments
Some data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease (AD). For up-to-date information, see www.adni-info.org. R.F.B. is funded with the NHMRC Dementia Research Fellowship (APP1105576). J.S.R. is funded by the Canadian Institutes of Health Research Postdoctoral Fellowship. This work was supported with funding from the National Institutes of Health, including P01 AG036694 (Sperling and Johnson), P50 AG005134 (Sperling, Johnson, Hedden), K23 EB019023 (Sepulcre), K23 AG049087 (Chhatwal), K24 AG035007 (Sperling), and K01 040197 (Hedden). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers S10RR021110, S10RR023401, and S10RR023043. For ADNI, data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904), and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is also funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Financial disclosures: A.P.S. has been a paid consultant for Janssen Pharmaceuticals and Biogen. K.V.P. has been a paid consultant for Biogen. D.M.R. served as a consultant for Eli Lilly, Biogen Idec, and Lundbeck Pharmaceuticals and serves as a member of the Scientific Advisory Board for Neurotrack. K.A.J. has served as a paid consultant for Bayer, GE Healthcare, Janssen Alzheimer's Immunotherapy, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, ISIS Pharma, AZTherapies, GEHC, Lundberg, and AbbVie. He is a site coinvestigator for Lilly/Avid, Pfizer, Janssen Immunotherapy, and Navidea. He has spoken at symposia sponsored by Janssen Alzheimer's Immunotherapy and Pfizer. He receives funding from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497 and the Alzheimer's Association grant ZEN-10-174210. R.A.S. has served as a paid consultant for AbbVie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a coinvestigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen and receives research support from Janssen Pharmaceuticals and Eli Lilly and Co. These relationships are not related to the content in the manuscript. R.A.S. also receives research support from the following grants: P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, and R01 AG027435, Fidelity Biosciences, Harvard NeuroDiscovery Center, and the Alzheimer's Association.
Footnotes
*Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) and Australian Imaging Biomarkers and Lifestyle study of aging database (aibl.loni.usc.edu). As such, the investigators within the ADNI and AIBL contributed to the design and implementation of ADNI and AIBL and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI and AIBL investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf and https://aibl.csiro.au/about/aibl-research-team/
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.08.004.
Supplementary Data
References
- 1.Sperling R.A., Mormino E., Johnson K. The Evolution of Preclinical Alzheimer’s Disease: Implications for Prevention Trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 Study: Stopping AD Before Symptoms Begin? Sci Translational Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe C.C., Ellis K., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mielke M.M., Wiste H.J., Weigand S.D., Knopman D.S., Lowe V.J., Roberts R.O. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Wiste H.J., Weigand S.D., Knopman D.S., Vemuri P., Mielke M.M. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 2015;72:511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrotin A., Mormino E.C., Madison C.M., Hayenga A.O., Jagust W.J. Subjective cognition and amyloid deposition imaging: A Pittsburgh compound b positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley R.F., Villemagne V.L., Masters C.L., Ellis K.A., Rowe C.C., Johnson K. A conceptualization of the utility of subjective cognitive decline in clinical trials of preclinical Alzheimer’s disease. J Mol Neurosci. 2016;60:354–361. doi: 10.1007/s12031-016-0810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merrill D.A., Siddarth P., Saito N.Y., Ercoli L.M., Burggren A.C., Kepe V. Self-reported memory impairment and brain PET of amyloid and tau in middle-aged and older adults without dementia. Int Psychogeriatrics. 2012;24:1076–1084. doi: 10.1017/S1041610212000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snitz B.E., Lopez O.L., McDade E., Becker J.T., Cohen A.D., Price J.C. Amyloid-β Imaging in Older Adults Presenting to a Memory Clinic with Subjective Cognitive Decline: A Pilot Study. J Alzheimer's Dis. 2015;48:S151–S159. doi: 10.3233/JAD-150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J. Subjective Cognitive Complaints, Personality and Brain Amyloid-beta in Cognitively Normal Older Adults. The Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel J.W., Varga Doležalovα M., La Joie R., Marks S.M., Schwimmer H.D., Landau S.M. Subjective cognitive decline and β-amyloid burden predict cognitive change in healthy elderly. Neurology. 2017;89:2002–2009. doi: 10.1212/WNL.0000000000004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley R.F., Hanseeuw B., Schultz A.P., Vannini P., Aghjayan S.L., Properzi M.J. Region-specific association of subjective cognitive decline with tauopathy independent of global β-amyloid burden. JAMA Neurol. 2017;74:1455–1463. doi: 10.1001/jamaneurol.2017.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stomrud E., Hansson O., Blennow K., Minthon L., Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 16.Mosconi L., De Santi S., Brys M., Tsui W.H., Pirraglia E., Glodzik-Sobanska L. Hypometabolism and Altered Cerebrospinal Fluid Markers in Normal Apolipoprotein E E4 Carriers with Subjective Memory Complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwan M.D., Villemagne V.L., Doré V., Buckley R., Bourgeat P., Veljanoski R. Subjective Memory Complaints in APOEϵ4 Carriers are Associated with High Amyloid-β Burden. J Alzheimer's Dis. 2015;49:1115–1122. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]
- 18.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabin L.A., Smart C.M., Crane P.K., Amariglio R.E., Berman L.M., Boada M. Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures used Across 19 International Research Studies. J Alzheimer's Dis. 2015;48:S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aisen P.S., Petersen R.C., Donohue M.C., Gamst A., Raman R., Thomas R.G. Clinical Core of the Alzheimer's Disease Neuroimaging Initiative: progress and plans. Alzheimer's Demen. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis K.A., Bush A., Darby D., De Fazio D., Foster J., Hudson P. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 22.Dagley A., LaPoint M., Huijbers W., Hedden T., McLaren D.G., Chatwal J.P. Harvard Aging Brain Study: Dataset and accessibility. NeuroImage. 2017;144:255–258. doi: 10.1016/j.neuroimage.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farias S.T., Park L.Q., Harvey D.J., Simon C., Reed B.R., Carmichael O. Everyday Cognition in Older Adults: Associations with Neuropsychological Performance and Structural Brain Imaging. J Int Neuropsychol Soc. 2013;19:430–441. doi: 10.1017/S1355617712001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farias S.T., Mungas D., Reed B.R., Cahn-Weiner D., Jagust W., Baynes K. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go R.C., Duke L.W., Harrell L.E., Cody H., Bassett S.S., Folstein M.F. Development and validation of a structured telephone interview for dementia assessment (STIDA): the NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10:161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- 26.Amariglio R.E., Townsend M.K., Grodstein F., Sperling R.A., Rentz D.M. Specific Subjective Memory Complaints in Older Persons May Indicate Poor Cognitive Function. J Am Geriatr Soc. 2011;59:1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts R.O., Aakre J.A., Kremers W.K., Vassilaki M., Knopman D.S., Mielke M.M. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 2018;75:970–979. doi: 10.1001/jamaneurol.2018.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Joie R., Perrotin A., Egret S., Pasquier F., Tomadesso C., Mézenge F. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: Association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimer's & Dementia: Diagnosis. Assess Dis Monit. 2016;5:23–34. doi: 10.1016/j.dadm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley R.F., Mormino E.C., Amariglio R.E., Properzi M.J., Rabin J.S., Lim Y.Y. Sex, Amyloid, and APOEe4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimer's Demen. 2018;14:1193–1203. doi: 10.1016/j.jalz.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohman T., Dumitrescu L., Barnes L., Thambisetty M., Beecham G., Kunkle B. Sex-specific effects of Apolipoprotein E on cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75:989–998. doi: 10.1001/jamaneurol.2018.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosconi L., Berti V., Quinn C., McHugh P., Petrongolo G., Varsavsky I. Sex differences in Alzheimer risk. Brain Imaging Endocr vs chronologic Aging. 2017;89:1382–1390. doi: 10.1212/WNL.0000000000004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonker C., Geerlings M.I., Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Hülür G., Hertzog C., Pearman A.M., Gerstorf D. Correlates and Moderators of Change in Subjective Memory and Memory Performance: Findings from the Health and Retirement Study. Gerontology. 2015;61:232–240. doi: 10.1159/000369010. [DOI] [PubMed] [Google Scholar]
- 36.Wolfsgruber S., Polcher A., Koppara A., Kleineidam L., Frölich L., Peters O. Cerebrospinal fluid biomarkers and clinical progression in patients with subjective cognitive decline and mild cognitive impairment. J Alzheimer's Dis. 2017;58:939–950. doi: 10.3233/JAD-161252. [DOI] [PubMed] [Google Scholar]
- 37.Snitz B.E., Wang T., Cloonan Y.K., Jacobsen E., Chang C.-C.H., Hughes T.F. Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimer’s & Dementia. 2018;14:734–742. doi: 10.1016/j.jalz.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley R.F., Ellis K.A., Ames D., Rowe C.C., Lautenschlager N.T., Maruff P. Phenomenological characterization of memory complaints in preclinical and prodromal Alzheimer’s disease. Neuropsychology. 2015;29:571. doi: 10.1037/neu0000156. [DOI] [PubMed] [Google Scholar]
- 39.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's Demen. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.