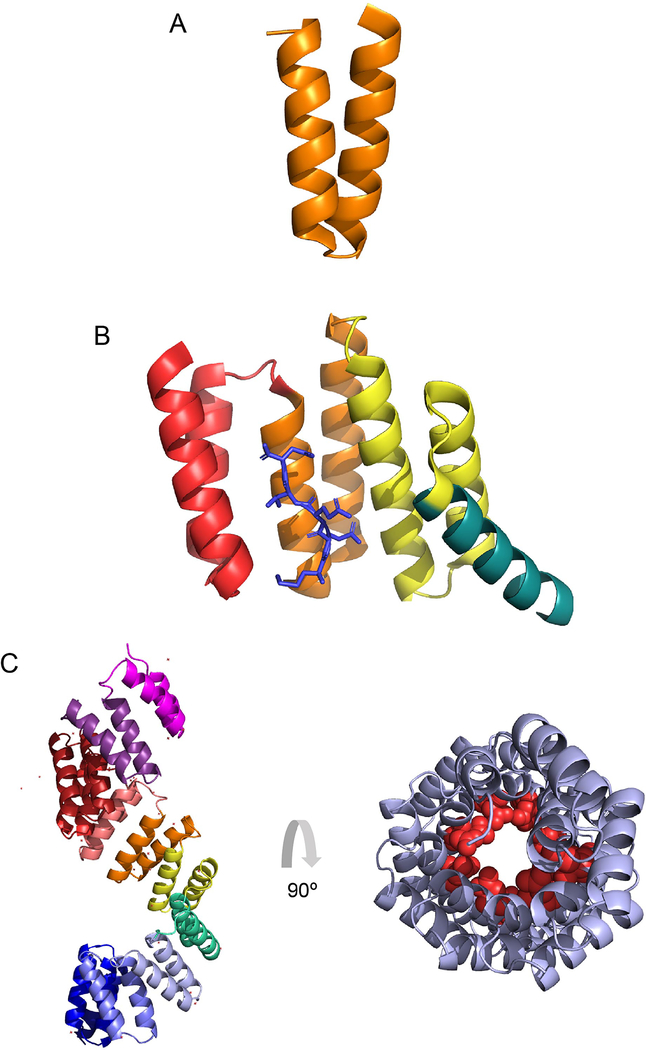
Figure 1. Tetratricopeptide repeat structures.
(A) A single tetratricopeptide repeat (TPR) motif (orange) consists of 34 amino acids that folds into two anti-parallel alpha helices. (B) TPR motifs are commonly found in clusters of three. The crystal structure (1ELR) represents the TPR domain of Hsp70/Hsp90 Organizing Protein (HOP), which binds to the chaperone Hsp90. The single TPR motifs are indicated in red, orange and yellow, with the C-terminal capping helix in turquoise. The C-terminal heptamer of Hsp90, which is the ligand for this cluster, is shown in blue. (C) The crystal structure (1W3B) represents the TPR region of OGT. It forms a superhelix with individual motifs indicated in pink, purple, brick red, red, orange, yellow, green, periwinkle, blue and violet from N-terminus to C-terminal, respectively (left panel). The structure in (C-right panel) is the 11.5 TPR motifs from (C- left panel) rotated 90 degrees, which demonstrates the superhelical nature of the tandem repeats. Conserved Asn (red spheres), thought to mediate target protein interaction, as seen in importin-α, line the inner surface of the superhelix.