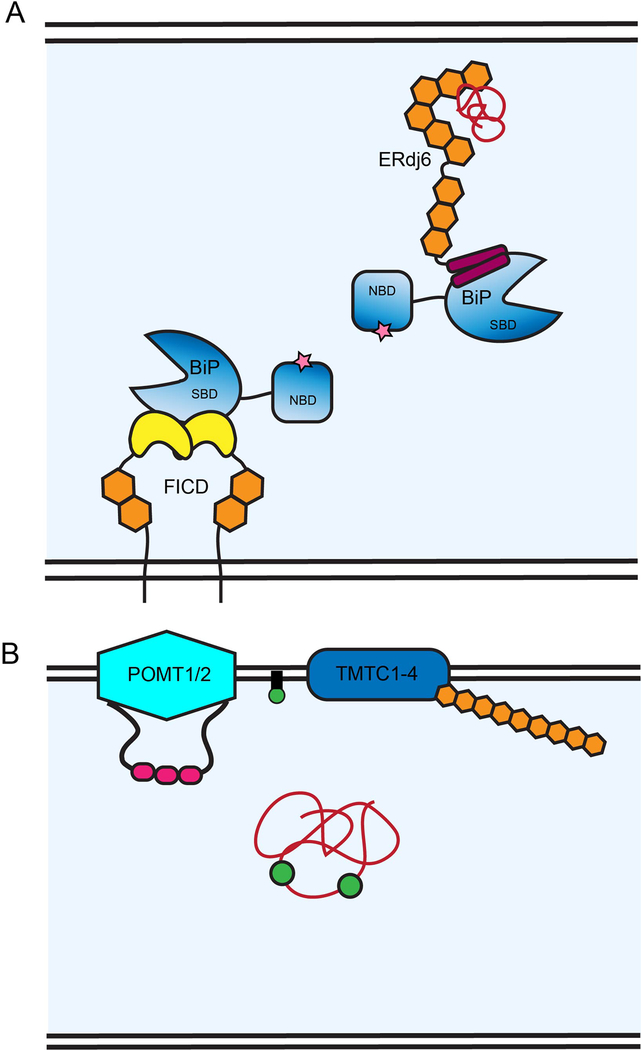
Figure 3. Sec72, SGTA and SEL1L are involved in ER protein translocation.
(1) Sec72 (orange) is anchored to the ER membrane by Sec71 (dark blue) and Sec63 (green) at the Sec61 (purple) translocon. The TPR domain, comprised of three TPR motifs (orange hexagons), binds the C-terminus of the yeast Hsp70 (blue), Ssa1, aiding in the recruitment of translated polypeptides (red) to the Sec61 translocon. (2) SGTA (orange) aids in post-translational translocation, specifically of tail-anchored proteins. SGTA is comprised of an N-terminal UBL-binding domain, which is also responsible for homo-dimerization, a middle TPR domain and C-terminal substrate binding domain that captures the tail-anchored proteins (light grey). SGTA is in a complex with UBL4A and Bag6 (dark and light green, respectively) and plays a role in molecular triage. This complex is responsible for the proper targeting of tail-anchored proteins to the ER membrane as well as segregating misfolded polypeptides and targeting them for degradation. As chaperones aid in this process, SGTA’s TPR domain is responsible for chaperone recruitment. The TPRs bind the C-termini of both Hsp70 and Hsp90 as well as proteasomal subunit, Rpn13. (3) As nascent polypeptides are queried for folding status, properly folded proteins are distinguished for export at ER exit sites (ERES) and misfolded proteins are targeted for degradation by ERAD. SEL1L (orange) is in complex with HRD1, the ER retro-translocon responsible for translocating misfolded proteins across the ER membrane for subsequent degradation by the proteasome (yellow) in the cytoplasm. SEL1L contains 11 SLR motifs (hexagons #1–11 from N to C-terminus), which are very similar to TPR motifs but with an extended consensus sequence length, that comprise the majority of the luminal portion of the protein. The SLR motifs nearest the membrane (#10–11) interact with a luminal portion of HRD1 and are necessary for interaction with ERAD factor, OS9 (brown). A SLR motif in the middle region (#9) is responsible for homo-oligomerization of SEL1L.