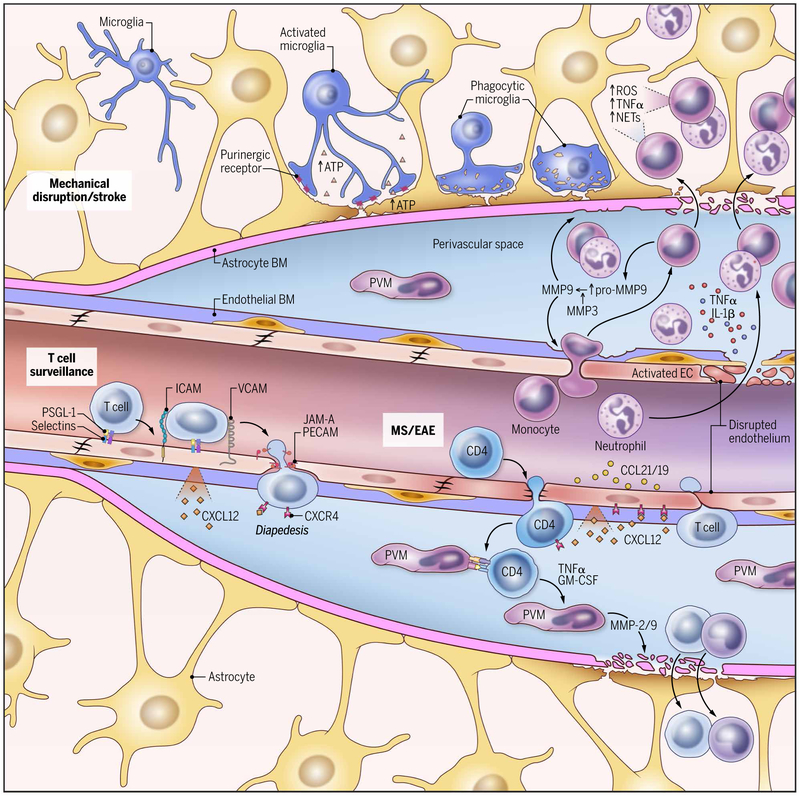
Fig. 4. Blood-CNS interface during inflammation.
This schematic of a CNS blood vessel summarizes three different immunological scenarios: steady-state T cell surveillance, CNS autoimmune disease, and cerebrovascular injury and stroke. T cell surveillance: During steady state, leukocyte extravasation across the BBB is limited to few activated T lymphocytes that interact with ICAM-1 and VCAM-1 expressed on the lumen of vascular endothelial cells. CXCL12 expression by endothelial cells on the abluminal side contributes to sequestering these activated CD4+ T cells in the perivascular space through binding to CXCR4 on the T cell. EAE: During CNS autoimmune diseases such as EAE, the efficiency of leukocyte diapedesis is increased. CCL19, CCL21, and CXCL12 are up-regulated by cerebrovascular endothelial cells that promote the recruitment and adhesion of encephalitogenic CD4+ T cells. Vascular adherence and extravasation are also facilitated by selectins (P-selectin–PSGL-1 interactions) and integrins (LFA-1–ICAM-1 and VLA-4–VCAM-1 interactions). CXCR7, expressed on the abluminal surface of endothelial cells, binds to CXCL12 and reduces T cell sequestration in the perivascular space. After extravasation, T cells interact with APCs, including perivascular macrophages (PVMs), in the perivascular space. Recognition of cognate-peptide MHC complexes results in production of chemokines and cytokines (such as TNFα and granulocyte-macrophage colony-stimulating factor) that promote recruitment of myelomonocytic cells from the blood. This is followed by production of metalloproteinases (such as MMP-2 and MMP-9) that selectively cleave dystroglycan in the astrocytic foot processes, allowing penetration of effector T cells into the CNS parenchyma. Vascular injury and stroke: Mechanical disruption of the glia limitans leads to a rapid release of ATP that is detected by purinergic receptors expressed on microglia. The microglia provide immediate barrier support and debris clearance. Cerebrovascular injury can also cause resident and infiltrating monocyte-derived macrophages to release cytokines (TNFα and IL-1β), chemokines, ROS, and metalloproteinases (pro–MMP-9). TNFα and IL-1β trigger endothelial cell activation, promoting further myelomonocytic cell invasion. Pro–MMP-9 becomes activated by MMP-3, causing additional destruction of the glia limitans. Once in the parenchyma, neutrophils can release ROS and NETs in an attempt to control pathogens that are not present. These effector mechanisms contribute to tissue damage.