Figure 10.
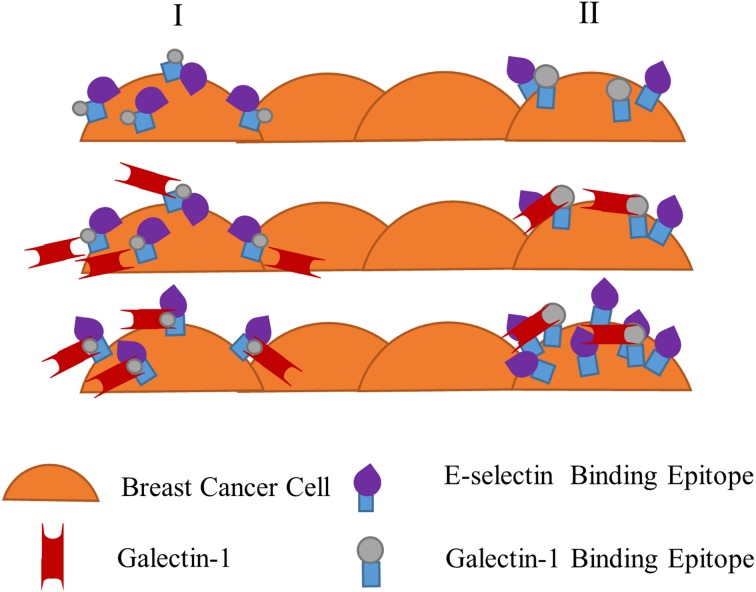
Hypothesized effects of Gal-1 on E-selectin receptor-ligand binding interaction with a monolayer of breast cancer cells. Two hypothesized pathways, column I and column II, of Gal-1’s effect on E-selectin receptor-epitope interaction. Column I proposes E-selectin binding epitopes (sialofucosylated moieties such as sialyl Lewis X and sialyl Lewis A) are hidden or the entire E-selectin ligand is in a less active conformation on breast cancer cells until Gal-1 binds to N-Acetyllactosamine (minimal Gal-1 binding epitope) on the ligand. In this shared ligand model, Gal-1 binding leads to a conformational change, exposing E-selectin-reactive portions on the same ligand. Breast cancer cells are then able to bind more effectively to E-selectin at the lower range of physiologically-relevant fluid shear stress. Column II alternatively proposes that Gal-1 and E-selectin epitopes are expressed on distinct but similarly structured ligands. Gal-1 binding to its recognition epitope on one type of ligand induces a signal in the breast cancer cell to express more reactive E-selectin epitopes on another distinct ligand. Once again, enhanced binding of breast cancer cells to E-selectin occurs, particularly at low shear stress.