Abstract
The development of an endothelialized membrane oxygenator requires solution strategies combining the knowledge of oxygenators with endothelial cells’ biology. Since it is well known that exposing cells towards pure oxygen causes oxidative stress, this aspect has to be taken into account in the development of a biohybrid oxygenator system. N-Acetylcysteine (NAC) is known for its antioxidant properties in cells. We tested its applicability for the development of an endothelialized oxygenator model. Cultivating human umbilical vein derived endothelial cells (HUVEC) up to 6 days with increasing concentrations of NAC from 1 to 30 mM revealed NAC toxicity at concentrations from 20 mM. Cell density clearly decreased after radical oxygen species exposure in non-NAC pretreated cells compared to 20 mM NAC precultured HUVEC after 3 and 6 days. Also the survival rate after ROS treatment could be restored by incubation with NAC from 15 to 25 mM for all time points. NAC treated cells changed their morphology from typical endothelial cells’ cobblestone pattern to a fusiform, elongated configuration. Transformed cells were still positive for typical endothelial cell markers. Our present results show the potential of NAC for the protection of an endothelial cell layer in an endothelialized membrane oxygenator due to its antioxidative properties. Moreover, NAC induces a morphological change in HUVEC similar to dynamic cultivation procedures.
Keywords: Tissue engineering, Extracorporeal membrane oxygenation, ECMO, Extracorporeal carbon dioxide removal, ECCO2R, Oxygenator
Introduction
The application of an extracorporeal membrane oxygenator (ECMO) in end stage respiratory diseases such as cystic fibrosis as a bridge to transplant option allows the partial and temporary replacement of lung function.7 In acute respiratory distress syndrome (ARDS) ECMO allows a more protective regime of mechanical ventilation that leads to improved mortality in specialized centers.22 The mid- to long-term application of ECMO is currently forestalled by its limited hemocompatibility, leading to a significant loss of gas transfer capacity caused by unspecific protein absorption and thrombus formation, plasma leakage and leucocyte activation induced by the interaction of blood components with the foreign surface of the gas permeable membrane.1,2,23,26,27
The approach of coating the gas permeable membrane with endothelial cells to create a fully hemocompatible surface has been proposed.13,8 In pursuing this goal, a pertinent aspect of ventilation in ARDS is the hyperoxic acute lung injury (HALI) induced by hyperoxic mechanical ventilation.15 This must be taken into account, as a cellularized oxygenator will expose endothelial cells to pure oxygen.
Long-term oxygen exposure causes generation of radical oxygen species (ROS), which lead to a cellular redox imbalance and decompensation of the cellular antioxidant system such as consumption of the antioxidant glutathione (GSH).5,11
Excessive ROS levels, such as in inflammation or hyperoxic atmosphere, can induce apoptosis or even necrosis by lipid peroxidation or direct DNA damage.4 Proinflammatory signaling pathways such as the NF-κB pathway are activated resulting in increased leukocyte adhesion and platelet activation, which has to be strictly prevented in an endothelialized oxygenator device.4 – 21
On the other hand, superoxide is essential in endothelial cell proliferation and migration during angiogenesis whereas antioxidants were shown to be able to prohibit the same.18,3 These findings highlight the heterogeneous character of ROS in the function of endothelial cells.
Nevertheless, the impact of harmful long-term oxygen exposition towards the endothelial cell layer in a biohybrid oxygenator contrasts patients’ oxygen needs, if there is no viable option to restore the cellular antioxidant self-defense.
N-Acetylcysteine (NAC) is known as ROS scavenger, originally developed as a mucolytic drug. NAC acts antioxidatively via two pathways: On the one hand it deoxidizes ROS directly with its potent reductive thiol group. On the other hand NAC was shown to stimulate GSH synthesis and the GSH recycling procedure in endothelial cells.5,11 On the basis of its property to raise cellular GSH stock, NAC is used in patients with acetaminophen poisoning, in which liver cells’ GSH stock is utilized, leading to severe acute liver failure.12 Regarding endothelial cells, NAC was shown to protect pulmonary endothelial cells towards oxygen induced injury in a rat model by strengthening the cellular antioxidant system.24
We investigated the potential of N-Acetylcysteine to protect an endothelial cell layer from oxygen toxicity. Therefore we evaluated NAC toxicity on endothelial cells and tested confluent HUVEC cell layers precultured with different NAC concentrations in vitro for up to 6 days with respect to their resistance to an ROS former.
Methods
We cultivated pooled, human umbilical vein derived endothelial cell layers (HUVEC) from three different donors with different NAC concentrations in order to evaluate a potential harmful effect on proliferation. In a second trial we exposed HUVEC to a ROS former, to determine a possible protective effect in NAC supplemented cultivation. We analyzed the proliferation via an XTT-assay, measured the influence of NAC and ROS on cell survival and calculated cell density as a parameter reflecting cell layer’s integrity on days 1, 2, 3 and 6. Subsequently cells were immunocytochemically stained for common endothelial cell markers.
HUVEC Isolation
HUVEC were obtained from kindly donated umbilical cords (UC) from the Clinic of Gynecology and Maternity of the University Hospital of RWTH Aachen (vote of the local ethics committee: EK 019/16). UC were transferred into sterile transport buffer 100 mM HEPES, 140 mM NaCl, 2.5 mM KCl, 10 mM glucose, 1% antibiotic/antimycotic solution (penicillin G-streptomycin-amphotericin B; Gibco, Germany; pH 7.4). Before cells were dissolved from subendothelial connective tissue using 1% collagenase (Sigma, Germany) for 30 min at 37 °C, the umbilical vein was cannulated from both sides and carefully flushed with sterile PBS. Dissolved cells were resuspended in endothelial-basal medium supplemented with EGM-2 supplements (Lonza, Germany) and seeded into 2% gelatin pre-coated cell culture flasks (T175, Greiner, Germany) at a cell density of 5 × 104 cells/cm2. Medium was changed every three days. Upon 70–80% confluence, HUVEC were subcultivated using 0.25% trypsin/0.02% EDTA solution (Gibco, Germany).
Cryoconservation and Thawing
HUVEC were cryoconserved in second passage using freezing medium [60% DMEM, 20% FCS (both Gibco, Germany), 20% Dimethylsufoxide (DMSO, Sigma, Germany)] in an equal ratio to cell suspension. Cryotubes were maintained in a freezing container (Mr. Frosty™; Thermo Scientific, USA) at room temperature for 30 min. Afterwards cells were cooled down to −80 °C for 24 h until they were transferred into a nitrogen tank for definite storing. For thawing cryotubes were warmed up to 37 °C immediately before the cell suspension was transferred into 10% FCS containing DMEM. After resuspending HUVEC in EGM–2, cells were seeded at a density of 5 × 104 cells/cm2 into 2% gelatin pre-coated culture flasks (T175, Greiner). Cells in passage 4 were used.
Cell Survival and Density
To analyze cell survival and density after NAC exposure, 104 HUVEC were seeded into gelatin-coated wells of 96-well plates (Greiner, Germany). EGM-2 medium was changed every day and cells cultured to confluence. Consequently, the cell culture medium was enriched with NAC in seven different concentrations (1, 5, 10, 15, 20, 25, 30 mM). As a control we used EGM-2 without NAC supplementation. An NAC stock solution was made in EGM-2 and acid pH-shift was buffered with NaOH. Stock solution was sterilely filtered using a syringe filter (0.2 µm pore size, Corning, Germany). Thereafter a NAC dilution series in EGM-2 was done, and 100 µL of the respective NAC supplemented cell culture medium was added to each well. Endothelial cells were incubated with the NAC supplemented medium for 1, 2, 3 and 6 days.
In order to evaluate a potentially protective effect of NAC in ROS treated HUVEC, cells were exposed to 200 µM ROS generator tert-Butylhydroperoxid (TBHP, Luperox®; Sigma) for 30 min after 1, 2, 3 and 6 days of NAC supplemented cultivation.
The following assay was accomplished with untreated and TBHP-treated cells incubated with 8 different NAC concentrations. The cell survival was measured by calcein-AM and propidium iodide staining. Cells were incubated with calcein-AM (0.5 µM, R&D Systems) for 30 min to stain living cells. Afterwards, dead cells were stained with propidium iodide (1 µg/mL, R&D Systems) and images of the wells acquired directly with a fluorescent microscope (AxioObserver Z1, Carl Zeiss) with a high resolution CCD camera (AxioCam MRC, Carl Zeiss). Automated cell counting was performed using CellProfiler™ imaging software with a reproducible pathway, ensuring equal calculation proceedings. Cell survival was then calculated normalized to the respective control of the same time point. Cell density was calculated with the absolute cell numbers of living cells per square millimeter (mm2).
NAC Toxicity Testing
On days 1, 2, 3 and 6 the cells were washed twice with PBS (as NAC reacts with the XTT reagent). Then, EGM-2 with XTT-test reagent (Cell Proliferation Kit II, Sigma) was added. Absorbance was measured after 4 h at 475 nm with a TECAN plate reader.
Immunocytochemistry
HUVEC were fixed with ice-cold methanol for 10 min and rinsed with PBS subsequently. Unspecific binding sites were blocked using PBS (+3% BSA) for 45 min. Primary antibody mouse-anti-CD31 (Sigma P8590, 1:100) was added. Subsequently after rinsing again, cells were incubated humidified with secondary antibody goat-anti-mouse (Invitrogen, Alexa Fluor 488, A11001, 1:400). After washing cells, now using permeabilizing washing buffer (0.1% Triton in PBS), staining procedure for intracellular markers was undertaken using primary antibody rabbit-against vWF (Dako, A0082, 1:100) with secondary antibody goat-anti-rabbit (Alexa Fluor 594, Invitrogen A11012, 1:400) and primary antibody mouse against αSMA (Sigma, A2547) with secondary antibody goat-anti-mouse (Invitrogen, Alexa Fluor 594, A11005). Incubation time for every antibody was 60 min at 37 °C. Cells were counterstained with DAPI. Negative control was done using only secondary antibody.
Microscopy
Immunocytochemically stained HUVEC and nuclear staining for cell counting were imaged with a fluorescent microscope. Images were acquired using a high resolution CCD camera. Live HUVEC culture was evaluated with a Live Cell Imaging Microscope (Axio Zoom.V16, Carl Zeiss). Images were acquired with a CCD camera (AxioCam MRm, Carl Zeiss).
Statistics
Continuous variables are expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). Two-way ANOVA tests were performed to evaluate statistical significance. For XTT-assay and survival Dunnett’s and for cell density Sidak’s multiple comparisons tests were used. The respective p-values are shown in the diagrams. Data analysis was performed using commercially available software (GraphPad Prism 7, 2016, USA).
Results
NAC Toxicity
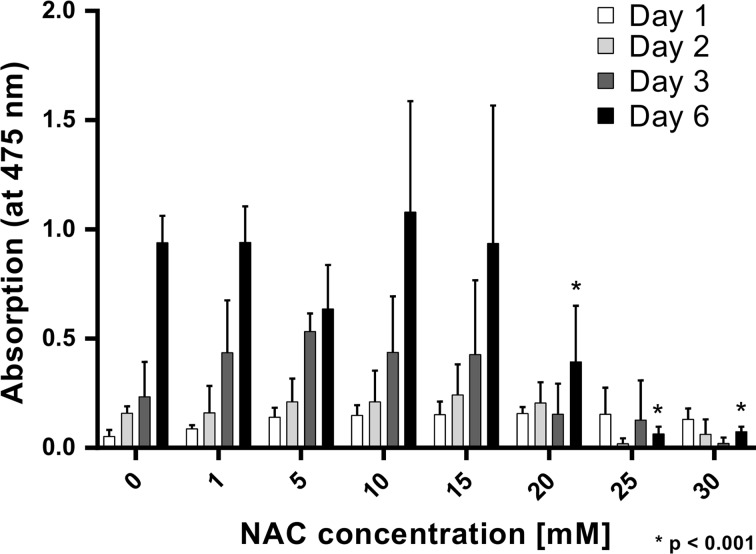
In Fig. 1 the proliferation rates of cells incubated with different concentrations of NAC is shown. After 24, 48 and 72 h, no significant influence of either NAC concentration is seen. The proliferation is significantly reduced only for 20, 25 and 30 mM NAC on day 6.
Figure 1.
NAC Toxicity (XTT-Test). Light absorption at a wavelength of 475 nm represents the proliferation of cells cultured with NAC supplemented EGM-2 medium. A significant harmful effect on HUVEC proliferation was observable ≥20 mM NAC on day 6 only (* p < 0.001).
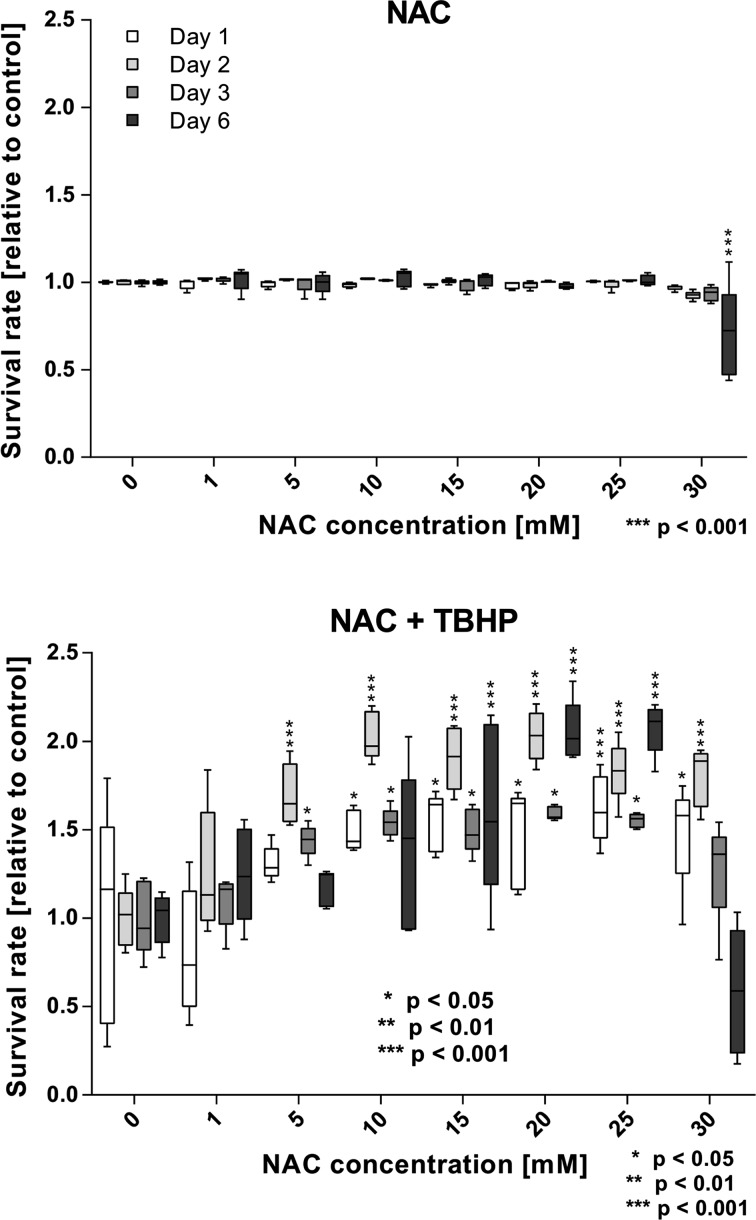
Survival of cells exposed to NAC did not differ significantly from the control for concentrations from 1 to 25 mM for all time points (Fig. 2). A significant drop in cell survival when compared to the untreated control was only observed for a NAC concentration of 30 mM after 6 days (70.57 ± 23.83% compared to the respective control, Fig. 2).
Figure 2.
Cell Survival of NAC and NAC + TBHP-treated cells. Top: Cell survival is reduced for 30 mM NAC on day 6 only. Bottom: NAC incubation is able to restore TBHP toxicity for concentrations from 5 mM NAC. In long term, only 15–25 mM increase cell survival significantly (* p < 0.05, ** p < 0.01, *** p < 0.001).
ROS Exposition
NAC pre-cultivated confluent HUVEC cell layers were exposed to the ROS generator TBHP to induce cellular oxidative stress. The cell survival significantly increased for cells treated with 15–25 mM NAC for all time points (Fig. 2). On days 1 and 2 also 30 mM NAC significantly increased the survival rate. For concentrations of 5 and 10 mM NAC, a significantly higher survival was also seen at shorter incubation periods, however not for 6 days.
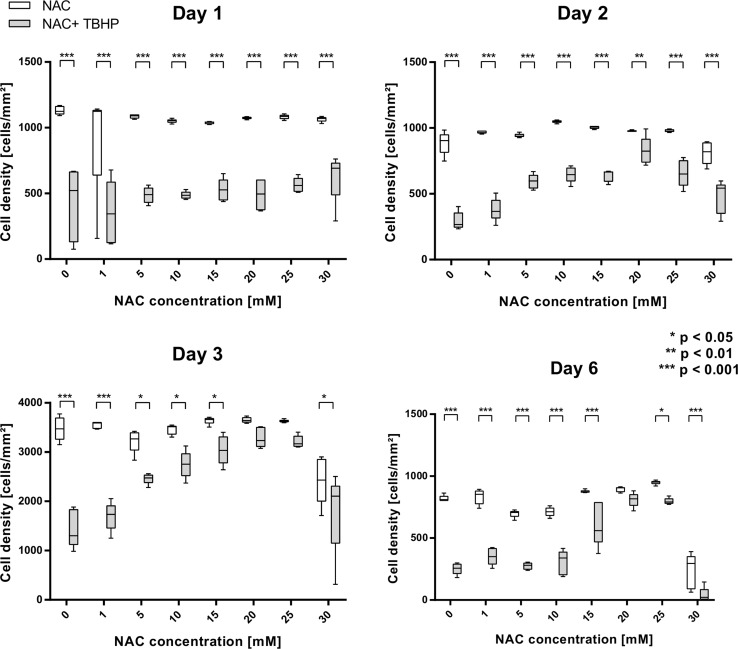
Cell densities are shown in Fig. 3. Endothelial cells without NAC enriched precultivation showed a significant loss of cell density after TBHP application on all days. After 24 and 48 h, the cell density is significantly reduced for all NAC concentrations. After 3 and 6 days, a similar cell density is seen between untreated and TBHP-treated cells for concentrations of 20 and 25 mM and only 20 mM NAC, respectively.
Figure 3.
Cell density of NAC and NAC + TBHP-treated cells. Top: On days 1 and 2, TBHP decreases the density significantly for all NAC concentrations. Bottom: NAC concentrations of 20 and 25, or only 20 can avoid TBHP toxicity after 3 and 6 days, respectively (* p < 0.05, ** p < 0.01, *** p < 0.001).
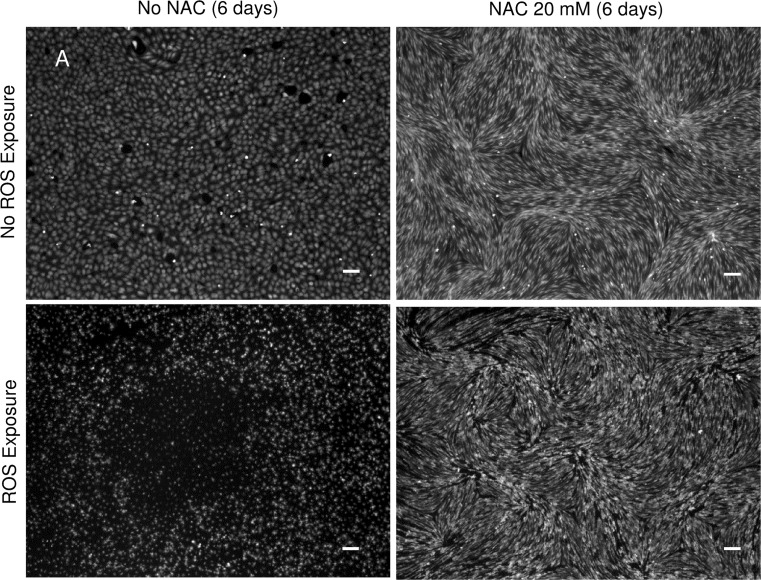
Figure 4 contains microscopic images of the Calcein staining; it displays a preserved integrity of the endothelial cell layer in the presence of NAC at a concentration of 20 mM when exposed to THBP after 6 days of precultivation.
Figure 4.
CalceinAM staining of NAC and NAC + TBHP-treated cells after 6 days of precultivation. NAC precultivation preserves the integrity of the endothelial cell layer in the presence of TBHP at a concentration of 20 mM (scale bar: 100 µm).
Immunocytochemical Characterization of NAC Exposed Cells
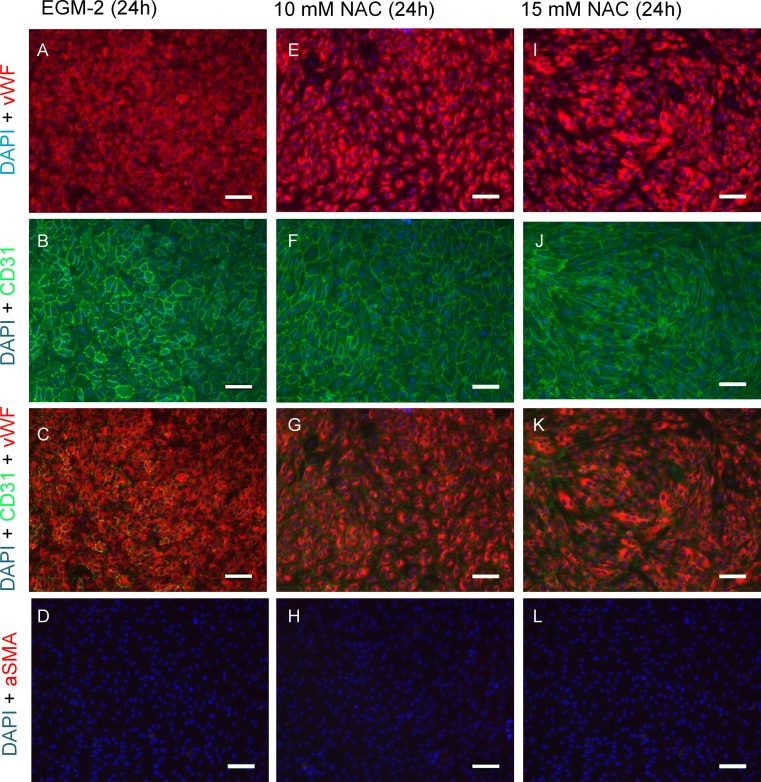
After NAC incubation period of 24 h endothelial cells changed their morphology from the typical cobble stone pattern, to an elongated, quasi-fusiform configuration with no observable intercellular gaps. A slight morphological rearrangement was already detectable at 10 mM NAC (Figs. 5e–5h) while a culture with 15 mM NAC lead to a marked shift in cell shape (Figs. 5i–5l). Cells were stained for endothelial markers CD31 (PECAM-1, Figs. 5b, 5f, 5j) and von-Willebrand-Factor (vWF, Figs. 5a, 5e, 5i) that exhibited typical expression patterns. Cells were shown to be negative for alpha smooth muscle actin (αSMA), a common marker of myofibroblasts. Morphological transformation in NAC treated cells was also observable in live cell imaging microscopy, displaying a healthy culture comparable with non-NAC enriched cultivation (Fig. 6).
Figure 5.
Immunocytochemical characterization. Confluent HUVEC cell layers after 24 h of NAC supplemented culture. In 15 mM NAC enriched cultivation, endothelial cells changed their morphology from typical cobblestone pattern (a–d) to an elongated, quasi-fusiform configuration (i–l). A slight cellular rearrangement was already detectable at 10 mM (e–h). Cells were positive for common endothelial cell markers CD31 (PECAM-1) and Von-Willebrand-Factor (vWF). Nuclei were counterstained with DAPI. All cells were negative for alpha smooth muscle actin (αSMA), a common myofibroblast marker (scale bar: 100 µm).
Figure 6.
Live cell imaging. Confluent HUVEC layer after 24 h of EGM-2 (a), 10 mM (b) and 15 mM (c) NAC-supplemented EGM-2 cultivation (scale bar: 100 µm).
Discussion
Oxygen toxicity is a well-known problem of mechanical ventilation in critically ill patients. We tested NAC for possible protective effects against reactive oxygen species on confluent HUVEC culture layers.
Our findings suggest that using NAC in cultivating a confluent HUVEC layer does not affect cell viability, reflected by cell survival, compared to non-NAC-supplemented cell cultivation at concentrations up to 25 mM. A concentration of 30 mM NAC in culture medium led to a significant decrease in cell survival. A study of Mukherjee et al. on the effect of the antioxidants NAC and Mitoquinone-Q on endothelial cells showed a significant higher level of oxidative stress in cells treated with 30 mM NAC compared to lower concentrations resulting in up-regulated expression of proinflammatory markers.20 This can explain the dose dependent toxicity of NAC observed here. Mukherjee et al. preincubated their endothelial cells only for 18 h. Our study systematically tested on the dose-dependent effect of NAC on HUVEC survival and density for different time points up to 6 days. Therefore, we consider this as an important step towards a long-term application of NAC with regard to cell viability.
By exposing HUVEC to the ROS generator TBHP, we investigated the effect of NAC pretreated HUVEC on oxidative stress resistance. Our results implicate that the pretreatment of a confluent HUVEC cell layer with 15–25 mM NAC supplemented EGM-2 significantly protects from ROS exposure in terms of immediate survival rates compared to untreated cells. The cell density reflects this finding from day 3. This suggests that the application of NAC in vitro can assure cell layer’s integrity although harmful ROS are disrupting culture. However, a short-term incubation with NAC is not sufficient. These findings are very well corresponding to the investigations of Sala et al. on the protective effect of NAC towards oxygen toxicity on pulmonary endothelial cells.24 However, with regard to clinical applicability by systemic administration, a target plasma concentration of 15 mM or more in steady state seems unlikely. Sae-Yong et al. tested the antioxidant effect of NAC towards fibroblasts that were ROS stimulated with paraquat. They determined an optimal concentration of 10 mM NAC in vitro. Their following in vivo study on the pharmacokinetic properties of NAC proposed, that achieving the desired target plasma concentration of 10 mM NAC would have meant a loading dose of 5010 mg/kg and a maintenance dose of 2250 mg/kg, which is clinically impossible due to severe side effects.14 These results illustrate the difficulty of transferring in vitro experiments on NAC to in vivo conditions, not impairing the acquired in vitro findings. A possible solution strategy might thus be a local drug delivery system that would allow high local concentrations of NAC similar to drug eluting coronary stents which can help avoiding adverse systemic effects.
To our knowledge, the change of HUVEC phenotype from cobblestone pattern to an elongated, fusiform configuration induced by NAC has not yet been described. As the elongated morphology of an endothelial cell represents the typical blood vessel lining phenotype—orientated in the direction of blood stream and with beneficial fluid-dynamic properties—the addition of NAC to culture medium might well facilitate cell seeding and preconditioning in an endothelialized oxygenator.6,25
Previously, relations between redox’ and cytoskeleton’s state in endothelial cells have been described: Levesque et al. showed that endothelial cells subjected to constant laminar flow displayed a lower proliferation rate compared to endothelial cells exposed to a disturbed flow pattern.17 This was underscored by the studies of Mitsumata et al. that showed a lower DNA synthesis rate in cells exposed to a constant laminar flow pattern.19 Endothelial cells from areas of constant laminar shear stress display a longitudinal phenotype; whereas cells from branch areas with a disturbed flow pattern were shown to be polygonal in shape.19,28 Consistent with these studies, proliferation activity was proven to be inducible by external ROS application in endothelial cells.18
Thus, we hypothesize that the application of NAC leads to a decreased cellular ROS level causing an elongated, lower-proliferating phenotype, which is typical for endothelial cells in blood vessels.
Thus far, achieving the elongated phenotype in endothelial cells was only possible using complex dynamic culture protocols in specialized bioreactors, reproducing physiological blood-stream conditions.9 The application of NAC in static cultivation conditions did not lead to a unidirectional cellular alignment, but rather to groups of vortices. This finding suggests the possibility of stimulating endothelial cells’ cytoskeleton, either to make dynamic culture procedures more effective or even to replace dynamic culture.
Conclusion
Our present results show the potential of the ROS scavenger N-Acetylcysteine in the development of an endothelialized membrane oxygenator to overcome the problem of oxygen toxicity in such a device. For the first time, this study demonstrates NAC under static conditions as an inducer of the elongated phenotype of HUVEC normally present in blood vessels. These results necessitate further investigations on the molecular background of this phenomena and the final applicability of NAC as a ROS scavenger in dynamic cultures or even in in vivo settings.
Acknowledgments
We thank Ting-Yi Yang for technical assistance.
Conflict of interest
Dr. Cornelissen is grant holder of the IZKF Project Number T12 that made this research possible. Mr. Plein, Dr. Thiebes, Dr. Finocchiaro, Mr. Hesselmann, Prof. Steinseifer and Prof. Jockenhoevel declare that they do not have any conflict of interest.
Funding
Supported by a Grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University (IZKF Project Number T12).
Ethical approval
Institutional review was obtained for the use of donated human umbilical cords (vote of the local ethics committee: EK 019/16). No further studies involving human subjects were carried out. The research did not involve animal studies.
Contributor Information
Tobias Plein, Phone: +49 241/80-85462, Email: tobias.plein@rwth-aachen.de.
Anja Lena Thiebes, Phone: +49 241/80-85462, Email: thiebes@ame.rwth-aachen.de.
Nicole Finocchiaro, Phone: +49 163/2355310, Email: nicole@finocchiaro.de.
Felix Hesselmann, Phone: +49 241/80-80540, Email: hesselmann@ame.rwth-aachen.de.
Thomas Schmitz-Rode, Phone: +49 241/80 87111, Email: smiro@ame.rwth-aachen.de.
Stefan Jockenhoevel, Phone: +49 241/80-89886, Email: jockenhoevel@ame.rwth-aachen.de.
Christian G. Cornelissen, Phone: +49 241/80-35512, Email: ccornelissen@ukaachen.de
References
- 1.Basmadjian D, Sefton MV, Baldwin SA. Coagulation on biomaterials in flowing blood: some theoretical considerations. Biomaterials. 1997;18:1511. doi: 10.1016/S0142-9612(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 2.Biglioli P, Cannata A, Alamanni F, Naliato M, Porqueddu M, Zanobini M, Tremoli E, Parolari A. Biological effects of off-pump vs. on-pump coronary artery surgery: focus on inflammation, hemostasis and oxidative stress. Eur. J. Cardiothorac. Surg. 2003;24:260. doi: 10.1016/S1010-7940(03)00295-1. [DOI] [PubMed] [Google Scholar]
- 3.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, D’Agostini F, De Flora S, Noonan DM, Albini A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab. Investig.: J Tech Methods Pathol. 1999;79:1151. [PubMed] [Google Scholar]
- 4.Cho KS, Lee EH, Choi JS, Joo CK. Reactive oxygen species-induced apoptosis and necrosis in bovine corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1999;40:911. [PubMed] [Google Scholar]
- 5.Cotgreave I, Moldeus P, Schuppe I. The metabolism of N-acetylcysteine by human endothelial cells. Biochem. Pharmacol. 1991;42:13. doi: 10.1016/0006-2952(91)90674-T. [DOI] [PubMed] [Google Scholar]
- 6.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981;103:177. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Guzman E, Hoopes CW, Zwischenberger JB. The evolution of extracorporeal life support as a bridge to lung transplantation. Asaio J. 2013;59:3. doi: 10.1097/MAT.0b013e31827461c2. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich M, Finocchiaro N, Olszweski S, Arens J, Schmitz-Rode T, Sachweh J, Jockenhoevel S, Cornelissen CG. ENDOXY: development of a biomimetic oxygenator-test-device. PLoS ONE. 2015;10:e0142961. doi: 10.1371/journal.pone.0142961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada R, Giridharan GA, Nguyen MD, Roussel TJ, Shakeri M, Parichehreh V, Prabhu SD, Sethu P. Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal. Chem. 2011;83:3170. doi: 10.1021/ac2002998. [DOI] [PubMed] [Google Scholar]
- 10.Freedman JE. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008;28:s11. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 11.Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir. Med. 1998;92:609. doi: 10.1016/S0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- 12.Heard KJ. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med. 2008;359:285. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess C, Wiegmann B, Maurer AN, Fischer P, Moller L, Martin U, Hilfiker A, Haverich A, Fischer S. Reduced thrombocyte adhesion to endothelialized poly 4-methyl-1-pentene gas exchange membranes: a first step toward bioartificial lung development. Tissue Eng. Part A. 2010;16:3043. doi: 10.1089/ten.tea.2010.0131. [DOI] [PubMed] [Google Scholar]
- 14.Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, Chung YH, Lee EM, Hwang SK. Effect of high-dose intravenous N-acetylcysteine on the concentration of plasma sulfur-containing amino acids. Korean J. Intern. Med. 2005;20:217. doi: 10.3904/kjim.2005.20.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir. Care. 2013;58:123. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ. Res. 1999;84:516. doi: 10.1161/01.RES.84.5.516. [DOI] [PubMed] [Google Scholar]
- 17.Levesque MJ, Nerem RM, Sprague EA. Vascular endothelial-cell proliferation in culture and the influence of flow. Biomaterials. 1990;11:702. doi: 10.1016/0142-9612(90)90031-K. [DOI] [PubMed] [Google Scholar]
- 18.Maulik N. Redox regulation of vascular angiogenesis. Antioxid. Redox Signal. 2002;4:783. doi: 10.1089/152308602760598927. [DOI] [PubMed] [Google Scholar]
- 19.Mitsumata M, Nerem RM, Alexander RW, Berk B. Shear-stress inhibits endothelial-cell proliferation by growth arrest in the G0/G1 phase of the cell-cycle. FASEB J. 1991;5:A527. [Google Scholar]
- 20.Mukherjee TK, Mishra AK, Mukhopadhyay S, Hoidal JR. High concentration of antioxidants N-acetylcysteine and mitoquinone-Q induces intercellular adhesion molecule 1 and oxidative stress by increasing intracellular glutathione. J. Immunol. 2007;178:1835. doi: 10.4049/jimmunol.178.3.1835. [DOI] [PubMed] [Google Scholar]
- 21.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. (Baltimore, MD: 1950) 2004;173:3589. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 22.Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, Killer H, Mugford M, Thalanany M, Tiruvoipati R, Truesdale A, Wilson A. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health. 2006;6:163. doi: 10.1186/1472-6963-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rungatscher A, Tessari M, Stranieri C, Solani E, Linardi D, Milani E, Montresor A, Merigo F, Salvetti B, Menon T, Faggian G. Oxygenator is the main responsible for leukocyte activation in experimental model of extracorporeal circulation: a cautionary tale. Mediat. Inflamm. 2015 doi: 10.1155/2015/484979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala R, Moriggi E, Corvasce G, Morelli D. Protection by N-acetylcysteine against pulmonary endothelial-cell damage induced by oxidant injury. Eur. Respir. J. 1993;6:440. [PubMed] [Google Scholar]
- 25.Sato M, Levesque MJ, Nerem RM. Micropipette aspiration of cultured bovine aortic endothelial-cells exposed to shear-stress. Arteriosclerosis. 1987;7:276. doi: 10.1161/01.ATV.7.3.276. [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Yue Y, Huang X, Meng D. Protein adsorption on blood-contact membranes. J. Membr. Sci. 2003;222:3. doi: 10.1016/S0376-7388(03)00313-2. [DOI] [Google Scholar]
- 27.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol. 2013;33:2130. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]