Abstract
Background
The early diagnostic of lung cancer plays an important role in the prognosis of surgical treatment among lung cancer patients. To evaluate the clinical application of multi‐tumor markers protein biochip in the diagnosis of lung cancer, 12 tumor markers were detected in patients with different stages of lung cancer.
Methods
Serum CA125, CA19‐9, Ferritin, CA15‐3, CA242, CEA, AFP, NSE, PSA, f‐PSA, HGH, and β‐HGH were assessed in 506 patients, with 224 patients with lung cancer (including 123 cases of adenocarcinoma, 30 squamous cell carcinoma, 54 small‐cell carcinoma, and 17 non classification), 159 patients with benign lung disease and 90 healthy people control by the C‐12 multiple tumor protein‐chip detective system.
Results
The positive rate of C‐12 (77.23%) in lung cancer was significantly higher than that of benign lung disease (13.84%) and healthy people (9.76%) (P < .01). In lung cancer, the positive rate of CA199, NSE, CEA, CA242, Ferritin, f‐PSA, and CA125 were significantly higher than that of benign lung disease and healthy people. In adenocarcinoma, the positive rate of CA125 (73.53%) was significantly higher than that of squamous cell carcinoma (36.67%) and small‐cell carcinoma (56.62%).
Conclusion
The C‐12 multiple tumor protein‐chip detective system has acceptable sensitivity in the diagnostic of lung cancer.
Keywords: lung cancer, multi‐tumor markers protein biochip, tumor markers
1. INTRODUCTION
One of the most common malignant cancers namely lung cancer, with an estimated 1.8 million new cases merely occurring in 2012, accounts for about 13% of total cancer diagnoses throughout the world.1 In China, lung cancer accounts for most of the emerging cancers and is the leading cause of mortality of all cancers.2 Majority of patients have been diagnosed in the middle stage or late stage, and thus are unable to receive surgical treatment. Five years relative survival rate of lung cancer is <15% and patients with symptoms are even lower.3 However, the survival rate of patients with lung cancer may increase and the prognosis is obviously improved when surgical treatment is proceeding at early stage.4 Therefore, early diagnosis is essential for the improvement of the survival rate of lung cancer patients. There are several methods to make the diagnosis for lung cancer, such as chest radiographs, tumor markers, and pathological examinations. Radiographic inspection is an important test method for the diagnosis of lung cancer, but it is difficult to distinguish benign pulmonary nodules from malignant tumors.5 Pathological examination needs to take biopsy samples, which is invasive and has the risk of injury. Despite high sensitivity of pathological examination, false‐negative results may be obtained due to the limitation for the position from where the samples were taken.6
Tumor markers, commonly used as auxiliary index for diagnosing cancer, have been widely applied in clinical practice for many years.7 Compared with radiographs and pathological examination, tumor markers detections are convenient and inexpensive with fewer invasions. Several tumor markers, including carcinoembronic antigen (CEA), cytokeratin fragment 19 (CYFRA21‐1), human epididymis protein 4 (HE4), have been identified with considerable clinical significance for diagnosis and prognosis of lung cancer.8, 9, 10, 11 In actual fact, the sensitivity and specificity of single tumor marker detection is lower than the combined measurement of multiple tumor markers. The principal objective of the present study is to evaluate the predictive value of C‐12 multiple tumor marker protein‐chip detective system in lung cancer screening of apparently healthy populations and cancer patients.
2. MATERIALS AND METHODS
2.1. Patients and serum samples
Three groups of people were selected between January 2014 and December 2015 from the No. 150 Central Hospital of PLA. The first group included 224 lung cancer patients, with 151 men and 73 women. The ages of the 224 patients ranged from 23 to 92 years old, and the median age was 64.00 ± 12.62 years old. The diagnosis of each patient was confirmed by cytological diagnosis and/or immunehistochemistry (IHC) test. Patients with recurrence lung cancer were excluded. A total of 123 cases were adenocarcinoma (additionally including adenosquamous carcinoma), 30 cases were squamous cell carcinoma, 54 cases were small‐cell carcinoma, and 17 non classification. The second group was composed of 159 patients with benign pulmonary diseases, including pneumonia, tuberculosis, acute bronchitis, chronic bronchitis, bronchiectasis, and bronchial asthma that were confirmed by routine standard diagnostic methods or histological examination. Patients with a history of malignant disease, digestive or kidney disease, or 2 or more concomitant lung diseases were excluded. Of the 159 patients, 124 were men and 35 were women. The ages of the 159 patients ranged from 17 to 93 years old, and the median age was 62.01 ± 16.91 years old. The third group contained 123 people (78 men and 45 women) who attended in physical examination in outpatient during the same period, except for those with a family history of lung cancer. The ages of the 123 people ranged from 23 to 93 years old, and the median age was 60.12 ± 17.34 years old. This study was approved by the Ethics Committee of the No. 150 Central Hospital of PLA.
2.2. Sample collection and detection
A 2 mL fasting venous blood sample was collected from each patient in the morning into a sterile tube. After centrifugation at 716 g for 5 minutes, the supernatants were aspirated and then applied into detection or stored at 4°C within 7 days. The concentration of the multi‐tumor markers was quantified by a diagnostic kit (Huzhou HealthDigit corp.). The kit consisting of protein chip, concentrated washing buffer, reaction solutions, calibration solutions, control, solvent complex, and Test solution A & B. In particular, each of protein chips (namely, the reacting chips) includes 16 physically isolated subarrays whose layout is shown in Figure 1A. The samples were performed following the manufacturer's instructions with some modifications shown in Figure 2. The results were read by HD‐2001A/LU‐07 biochip reader (SHMY HealthDigit Biochips Co., Ltd. Shanghai, China).
Figure 1.
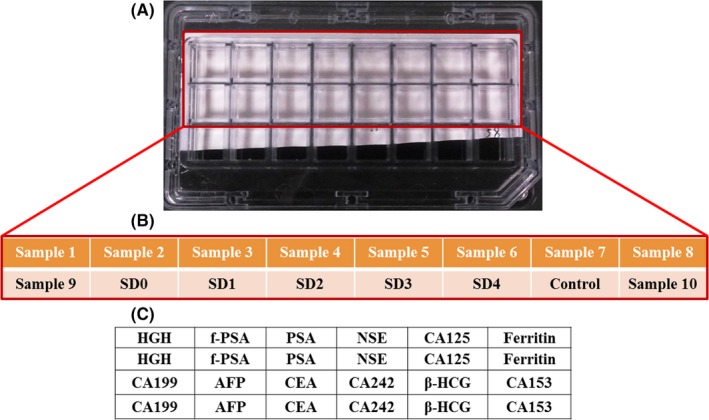
Typical layout of each chip and the designs of the subarrays. A, Layout of 1 agarose‐modified glass slide containing 16 physically isolated subarrays. B, B and A were one‐to‐one correspondence, and Samples 1‐16 were the serum samples to be tested, with SDs0‐4 as calibrator and Control as quality control serum in each chip. C, All the initial probes with 4 lines × 6 rows, including 12 antibodies (namely, anti‐HGH antibody, anti‐f‐PSA antibody, anti‐NSE antibody, anti‐CA125 antibody, anti‐Ferritin antibody, anti‐CA199 antibody, anti‐AFP antibody, anti‐CEA antibody, anti‐CA242 antibody, anti‐β‐HCG antibody, and anti‐CA153 antibody) were printed onto agarose‐modified glass slides in 2 replicates of a row and blocked, which were completed by the manufacturer before selling
Figure 2.
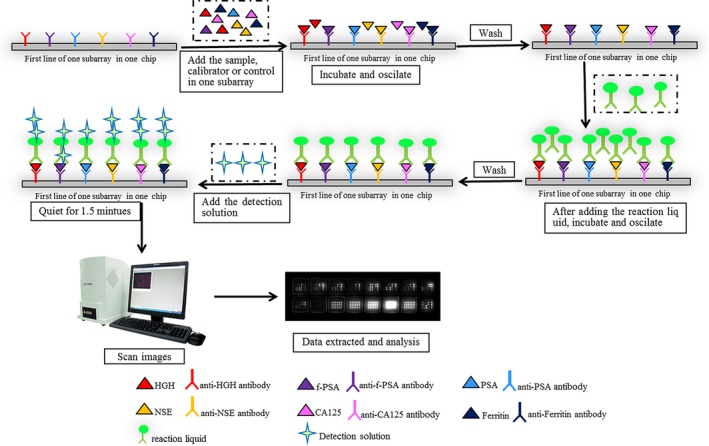
Procedure of the protein chip‐chemiluminescence method for simultaneous and rapid detection of 12 multi‐tumor markers and results interpreted
2.3. Detection indicator and normal reference range
Detection indicator and normal reference range were as follows: CA125 < 35 U/mL, CA199 < 35 U/mL, Ferritin < 219 ng/mL (female) and 322 ng/mL (male), CA153 < 35 U/mL, CA242 < 20 U/mL, CEA <5 ng/mL, AFP < 20 ng/mL, NSE < 13 ng/mL, PSA < 5 ng/mL, f‐PSA < 1 ng/mL, HGH < 7.5 ng/mL, and β‐HGH < 3 mIU/mL. Except Ferritin, if the tested result of any other tumor markers is higher than the normal reference range, the detected tumor marker can be defined as positive, otherwise negative.
2.4. Statistical methods and data analysis
Statistical analysis was performed using SPSS Statistics 19.0 (SPSS, Inc., Chicago, IL, USA). The difference of rate between groups was calculated by Chi‐square, and the level of serum markers between different lung cancers characteristics was compared by Student's t test. P < .05 was considered significant.
3. RESULTS
3.1. Comparison of the positive detection rates among the 3 groups
Positive rates of tumor markers among the 3 groups (namely lung cancer, hospitalized patients, and control) were included in the present study (shown in Table 1). The positive rate of the lung cancer group was 77.23%, the hospitalized patients’ group was 13.84% and the control group was 9.76%. Compared with the hospitalized patients and control group, the positive rate of the lung cancer group was significantly higher and calculated by χ2 test (χ2 = 239.1, P < .01; χ2 = 300.0, P < .01). The positive rate of CA199, NSE, CEA, CA242, Ferritin, and CA153 were significantly higher in patients with lung cancer than those with benign disease or apparently healthy people (P < .05), but not in β‐HCG, AFP, PSA, and HGH (P > .05). In addition, there was no significant difference in f‐PSA or CA125 between the cancer group and the hospitalized patients (P > .05).
Table 1.
Positive rate of total and C‐12 tumor markers in the 3 groups (%)
| Group | Number | Total positive rate (positive cases) | CA199 | NSE | CEA | CA242 | Ferritin | β‐HCG | AFP | f‐PSA | PSA | CA125 | HGH | CA153 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | 224 | 77.23 (180) | 37.05a | 9.82a | 61.16a | 21.07a | 17.41a | 3.13 | 4.46 | 4.91a | 3.13 | 70.98a | 0.45 | 10.71a |
| Hospitalized | 159 | 13.84 (22) | 18.87b | 3.77b | 35.22b | 9.43b | 22.01b | 1.26 | 2.52 | 5.66a | 4.40 | 74.21a | 0 | 5.66b |
| Control | 123 | 9.76 (12) | 4.76c | 0c | 2.38c | 0c | 0c | 0 | 0 | 0c | 2.38 | 2.38c | 0 | 0c |
In the same column, different number (eg, a and b, b and c, a and c) showed there was significant difference between the lung cancer, hospitalized and control group (P < .05). In the same column, the same number (eg, a and a, b and b, c and c) showed there was no significant difference between the lung cancer, hospitalized and control group (P > .05).
3.2. Serum level of C‐12 tumor markers among the 3 groups
Serum levels of the tumor markers are shown in Table 2. Compared with the hospitalized patients and control, serum levels of CA199, CEA, CA242, β‐HCG, AFP, CA125, and CA153 in cancer patients were significantly higher (P < .05). There was no significant difference in the concentration of β‐HCG, PSA, or HGH among the 3 groups (P < .05).
Table 2.
Serum level of C‐12 tumor markers in the 3 groups (x ± s)
| Group | Lung cancer (n = 224) | Hospitalized (n = 159) | Control (123) |
|---|---|---|---|
| CA199 (U/mL) | 91.84 ± 176.45a | 36.06 ± 87.11b | 11.17 ± 6.93c |
| NSE (ng/mL) | 8.65 ± 14.96a | 6.00 ± 5.33a | 3.74 ± 2.34c |
| CEA (ng/mL) | 38.12 ± 58.34a | 16.69 ± 33.75b | 2.02 ± 0.99c |
| CA242 (U/mL) | 28.39 ± 51.91a | 12.09 ± 30.05b | 4.39 ± 2.47c |
| Ferritin (ng/mL) | 172.29 ± 125.4a | 130.06 ± 129.20a | 92.64 ± 59.34c |
| β‐HCG (ng/mL) | 1.95 ± 12.72 | 2.47 ± 21.68 | 0.35 ± 0.13 |
| AFP (ng/mL) | 9.46 ± 28.93a | 4.29 ± 7.76b | 2.26 ± 1.12c |
| f‐PSA (ng/mL) | 0.28 ± 0.52a | 0.69 ± 4.46c | 0.22 ± 0.21a |
| PSA (ng/mL) | 1.71 ± 7.37 | 1.48 ± 3.48 | 1.23 ± 1.96 |
| CA125 (U/mL) | 158.91 ± 226.06a | 112.01 ± 132.56b | 12.74 ± 6.40c |
| HGH (ng/mL) | 0.62 ± 4.92 | 0.46 ± 0.77 | 0.28 ± 0.27 |
| CA153 (U/mL) | 14.84 ± 22.42a | 10.67 ± 13.15b | 8.14 ± 4.45c |
In the same row, different number (eg, a and b, b and c, a and c) showed there was significant difference between the lung cancer, hospitalized and control (P < .05). In the same row, the same number (eg, a and a, b and b, c and c) showed there was no significant difference between the lung cancer, hospitalized and control (P > .05).
3.3. Positive rates in different lung cancer based on cytological diagnosis and/or IHC
Positive rates of tumor markers among different lung cancer based on cytological diagnosis and/or IHC are shown in Table 3. In detail, the positive rates of adenocarcinoma, squamous cell carcinoma, and small‐cell carcinoma with joint detection were successively 69.92%, 60.00%, and 70.37%. Calculated by χ2 test, the positive rates of CEA, CA242, CA125, and CA153 in adenocarcinoma were apparently higher than that of squamous cell carcinoma, and the positive rate of CA125 was obviously higher than that of squamous cell carcinoma (P < .05).
Table 3.
Positive rate of total and C‐12 tumor markers in the group of the lung cancer (%)
| Group | Number | Total positive rate (%) | CA199 | NSE | CEA | CA242 | Ferritin | β‐HCG | AFP | f‐PSA | PSA | CA125 | HGH | CA153 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | 123 | 69.92 | 44.12 | 8.82 | 61.76a | 35.29a | 32.35 | 5.88 | 2.82 | 2.94 | 2.94 | 73.53a | 0 | 17.65a |
| Squamous cell carcinoma | 30 | 60.00 | 22.22 | 0 | 40.56b | 21.11b | 26.67 | 0 | 0 | 0 | 0 | 36.67b | 0 | 10.23b |
| Small‐cell carcinoma | 54 | 70.37 | 40.00 | 8.33 | 46.67b | 25.00b | 23.16 | 4.32 | 0 | 4.33 | 0 | 56.62c | 0 | 8.33b |
In the same column, different number (eg, a and b, b and c, a and c) showed there was significant difference between the adenocarcinoma, squamous cell carcinoma and small cell carcinoma group (P < .05). In the same column, the same number (eg, a and a, b and b, c and c) showed there was no significant difference between the adenocarcinoma, squamous cell carcinoma and small cell carcinoma group (P > .05).
3.4. Serum level of C‐12 tumor markers among 3 different lung cancers
Concentration of C‐12 tumor markers of 3 different lung cancer is shown in Table 4. It showed a significant increase in the concentration of CA199, CEA, CA242, and CA125 in adenocarcinoma compared with squamous cell carcinoma and small‐cell carcinoma (P < .05). In squamous cell carcinoma, the level of CA153 was higher than small‐cell carcinoma (P < .05).
Table 4.
Serum level of C‐12 tumor markers in the 3 groups (x ± s)
| Group | Adenocarcinoma (n = 123) | Squamous cell carcinoma (n = 30) | Small‐cell carcinoma (n = 54) |
|---|---|---|---|
| CA199 (U/mL) | 137.11 ± 196.70a | 68.88 ± 146.20b | 37.23 ± 28.55b |
| NSE (ng/mL) | 8.75 ± 12.57 | 5.51 ± 2.90 | 9.11 ± 6.02 |
| CEA (ng/mL) | 40.96 ± 54.10a | 22.68 ± 29.37b | 19.93 ± 25.81b |
| CA242 (U/mL) | 46.87 ± 66.57a | 14.40 ± 20.11b | 13.41 ± 12.04b |
| Ferritin (ng/mL) | 174.64 ± 133.65 | 223.66 ± 126.07 | 185.73 ± 97.71 |
| β‐HCG (ng/mL) | 3.16 ± 10.64 | 0.50 ± 0.40 | 8.21 ± 53.63 |
| AFP (ng/mL) | 9.28 ± 16.69 | 7.28 ± 6.56 | 4.64 ± 4.66 |
| f‐PSA (ng/mL) | 0.24 ± 0.20 | 0.12 ± 0.07 | 0.50 ± 0.69 |
| PSA (ng/mL) | 4.01 ± 17.76 | 0.55 ± 0.39 | 0.99 ± 0.41 |
| CA125 (U/mL) | 179.41 ± 227.12a | 80.46 ± 83.88b | 61.15 ± 81.03b |
| HGH (ng/mL) | 0.27 ± 0.32 | 0.35 ± 0.33 | 0.21 ± 0.11 |
| CA153 (U/mL) | 21.62 ± 37.43a | 19.85 ± 16.78a | 10.82 ± 13.47b |
In the same row, different number (eg, a and b, b and c, a and c) showed there was significant difference between the adenocarcinoma, squamous cell carcinoma and small cell carcinoma (P < .05). In the same row, the same number (eg, a and a, b and b, c and c) showed there was no significant difference between the adenocarcinoma, squamous cell carcinoma and small cell carcinoma (P > .05).
4. DISCUSSION
Lung cancer has been the most common and lethal malignant worldwide. Characteristic of the lung cancer patients presents with inoperable, advanced disease entailing a poor prognosis. Early diagnosis of lung cancer could significantly improve the response rate and prolong survival time of patients. Tumor markers are biochemical substances produced by tumor cells and present as intracellular substances in tissues or may be released into blood or body fluids.12 Detection of tumor markers has been widely used in cancer screen, evaluating the effectiveness of treatments and predicting prognostic information. In this study, the levels of tumor markers in serum were detecting using C‐12 multiple detection system. The positive rate of CA199, NSE, CEA, CA242, Ferritin, and CA153 was significantly higher in patients with lung cancer than those with benign disease or apparently healthy people. Ferritin is an iron‐storage protein and normally present in the serum and other body fluids. The level of Ferritin increased had been found in several malignancies, such as breast cancer, lymphoma, and metastatic disease.13, 14, 15 Hence, the level of ferritin may not be used to distinguish the lung cancer from the benign pulmonary. NSE is a subunit of enolase enzyme found mainly in neurons and neuroendocrine cells and is a marker of SCLC.16 CA125 is a glycoprotein found in epithelia ovarian carcinoma but not in normal adult ovarian tissues.17 In the diagnostic of lung cancer, it was reported that the sensitivity of CA125 was 44.25%.18 In the present study, the positive rate of CA125 was 70.98% in the lung cancer group, but in hospitalized patients, a high positive rate 74.21% was also observed. So, the CA125 may not be used as a valuable diagnostic marker for the screen of lung cancer with false positive. CEA is a glycoprotein that normally produced during fetal development but is not present in the blood of healthy people. Usually, CEA is considered to be a tumor marker in colorectal cancer or be associated with the prognostic of lung cancer.9 Recently, CEA has become the marker of choice for lung adenocarcinomas.19 In this study, the sensitivity of CEA was 61.16% in the lung cancer and 61.76% in lung adenocarcinomas. CA199 is a glycoprotein and exists as a ganglioside on tumor cell. CA199 is primarily used for the detection of cancer pancreas and gastrointestinal cancer.20, 21 It was reported that combination of CEA and CA199 can reach a sensitivity of 91.5% in the diagnostic of lung adenocarcinoma‐associated malignant pleural effusions (LA‐MPEs).22 In the present study, the sensitivity of the combination of CEA and CA199 was 69.64% in the lung cancer and 71.43% in adenocarcinoma.
In summary, the C‐12 multiple tumor protein‐chip detective system has acceptable sensitivity in the early screen for lung cancer, but cannot be used as a confirmed method.
Wang X, Zhang Y, Sun L, et al. Evaluation of the clinical application of multiple tumor marker protein chip in the diagnostic of lung cancer. J Clin Lab Anal. 2018;32:e22565 10.1002/jcla.22565
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Zhang WL, Wang Y, Han CZ. Characteristic trend analysis of cancer patients hospitalized in shanxi tumor hospital for the first time during 2001 and 2010. Asian Pac J Cancer Prev. 2015;16:3673‐3676. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8‐29. [DOI] [PubMed] [Google Scholar]
- 4. Klose J, Kobalz U. Two‐dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis. 1995;16:1034‐1059. [DOI] [PubMed] [Google Scholar]
- 5. Henschke CI, Yankelevitz DF. CT screening for lung cancer: update 2007. Oncologist. 2008;13:65‐78. [DOI] [PubMed] [Google Scholar]
- 6. Yang YJ, Cheng DY, Fang X, Li XX. The clinical diagnosis value of fibro‐optic bronchoscope examination combined with tumor marker determination to lung cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:312‐315. [PubMed] [Google Scholar]
- 7. Watanabe R, Takiguchi Y, Kuriyama T. Serum tumor markers for primary lung carcinoma. Nihon Rinsho. 2000;58:1070‐1073. [PubMed] [Google Scholar]
- 8. Ferrer J, Villarino MA, Encabo G, et al. Diagnostic utility of CYFRA 21‐1, carcinoembryonic antigen, CA 125, neuron specific enolase, and squamous cell antigen level determinations in the serum and pleural fluid of patients with pleural effusions. Cancer. 1999;86:1488‐1495. [DOI] [PubMed] [Google Scholar]
- 9. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138‐143. [DOI] [PubMed] [Google Scholar]
- 10. Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21‐1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45‐49. [DOI] [PubMed] [Google Scholar]
- 11. Zeng Q, Liu M, Zhou N, Liu L, Song X. Serum human epididymis protein 4 (HE4) may be a better tumor marker in early lung cancer. Clin Chim Acta. 2016;455:102‐106. [DOI] [PubMed] [Google Scholar]
- 12. Malati T. Tumour markers: an overview. Indian J Clin Biochem. 2007;22:17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs A, Jones B, Ricketts C, Bulbrook RD, Wang DY. Serum ferritin concentrations in early breast cancer. Br J Cancer. 1976;34:286‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs A, Slater A, Whittaker JA, Canellos G, Wiernik PH. Serum ferritin concentrations in untreated Hodgkin's disease. Br J Cancer. 1976;34:162‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gropp C, Havemann K, Lehmann FG. Carcinoembryonic antigen and ferritin in patients with lung cancer before and during therapy. Cancer. 1978;42:2802‐2808. [DOI] [PubMed] [Google Scholar]
- 16. Jorgensen LGM, Hirsch FR, Skov BG, Osterlind K, Cooper EH, Larsson LI. Occurrence of neuron specific enolase in tumor tissue and serum in small cell lung cancer. Br J Cancer. 1991;63:151‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neogi SS, Srivastava LM. Elevated tumor marker CA125: interpretations in clinical practice. Curr Med Res Pract. 2014;4:214‐218. [Google Scholar]
- 18. Diez M, Torres A, Maestro ML, et al. Prediction of survival and recurrence by serum and cytosolic levels of CEA, CA125 and SCC antigens in resectable non‐small‐cell lung cancer. Br J Cancer. 1996;73:1248‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non‐small cell lung cancer. Anticancer Res. 1999;19:3613‐3618. [PubMed] [Google Scholar]
- 20. Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients. Cell Biochem Biophys. 2015;71:1287‐1291. [DOI] [PubMed] [Google Scholar]
- 21. Zhong W, Yu Z, Zhan J, et al. Association of serum levels of CEA, CA199, CA125, CYFRA21‐1 and CA72‐4 and disease characteristics in colorectal cancer. Pathol Oncol Res. 2015;21:83‐95. [DOI] [PubMed] [Google Scholar]
- 22. Son SM, Han HS, An JY, et al. Diagnostic performance of CD66c in lung adenocarcinoma‐associated malignant pleural effusion: comparison with CEA, CA 19‐9, and CYFRA 21‐1. Pathology. 2015;47:123‐129. [DOI] [PubMed] [Google Scholar]


