Abstract
Background
Fecal calprotectin (FC) is non‐invasive inflammatory marker indicating various bowel diseases. However, the median‐specific cut‐off values and the standard deviations (SD) of the FC levels in each age group <4 years were not elucidated.
Methods
Healthy volunteers were enrolled from four kindergartens. A questionnaire was used to confirm that the children met the inclusion criteria, and several demographics and history of bowel symptoms were collected. The FC level was measured.
Results
A total of 234 healthy children aged between 6 months and 4 years were recruited. The median FC concentration of all participants was 245 μg/g (range 12–1033 μg/g, mean 68.5 μg/g, SD 123.12 μg/g). The children were divided into six age groups. The upper limit of 95% CI of median FC values was 135 μg/g in 7–12 months group, 65 μg/g in 13–18 months group, 55 μg/g in 19–24 months group, 40 μg/g in 25–30 months group, 21 μg/g in 31–36 months group, and 12 μg/g in 37–48 months group. A negative correlation trend was found between the age and the FC concentration.
Conclusion
This is the first study to present the FC median levels in the specific age groups <4 years in Korea. We found a FC level reduction with age, indicating a bowel maturation process and decreased intestinal permeability of the intestinal mucosa. In our study, FC levels reached the values of 50 μg/g around the age of 2 years.
Keywords: calprotectin, gut maturation, infant, reference values, South Korea
1. Introduction
Calprotectin is a 36‐kDa protein binding to calcium and other metal ions.1 The complex constitutes up to 60% of the soluble proteins in the cytosols of human neutrophils.2 In addition, it is distributed in monocytes, macrophages, and epithelial cells. Therefore, the level of calprotectin in feces can be proportional to the quantity of neutrophil migrated through the gastrointestinal mucosa. Neutrophil transepithelial migration and accumulation at the mucosal surface and within the gut lumen are a hallmark of digestive inflammatory pathology. In addition, its structure which contains calcium is very stable, and the high level of calcium in the intestine stabilizes the structure of calprotectin, which can be maintained in the feces for about 7 days.3 Fecal calprotectin (FC) may be an ideal routine screening tool for diagnosing the organic intestinal diseases because the simplicity and the sensitivity of the method. Elevated concentrations of FC have been described in inflammatory bowel diseases, such as Crohn's disease, ulcerative colitis (both in adults and in children), and can be used to evaluate the degree of inflammation in these patients.4, 5
In addition to gastrointestinal disorders, FC was recently reported as a reliable diagnostic method in cow's milk protein allergy and atopic diseases.6, 7, 8, 9, 10
For adults and children over 4 years, a cut‐off level of 50 μg/g has been well established for diagnostic purposes.4 Normally, a high level of calprotectin was reported in healthy neonates due to the higher intestinal permeability, the establishment of gut flora, and the response to alimentary antigens. Therefore, we suggest that different normal values of FC may be present in different age groups <4 years.
A few studies showed that children under the age of 4 years have higher FC values than older children and adults. Therefore, a cut‐off level of FC is necessary for this age group to routinely use the fecal calprotectin in clinical pediatric practice.
Therefore, the aim of this study was to measure the concentrations of FC in healthy children aged between 6 months and 4 years to find the reference values for FC in this age group in Korea.
Based on the results of this study, we hope to use this simple method in different settings of clinical pediatric practice.
2. Materials and Methods
2.1. Enrollment of subjects
To collect the feces from apparently healthy children (108 girls and 126 boys) aged between 0 and 4 years, four kindergartens in major cities or close to the urban areas of Korea over a 6‐month period were addressed.
The parents were contacted either personally or by flyers. All participants filled out a questionnaire about several demographic and clinical characteristics of their children.
2.2. Exclusion criteria
Children were excluded if their parents reported signs of a cold, flu, or stomach or similar problems in the last 2 weeks. In addition, children with a history of preterm birth, low or large birth weight, large or small weight for their age (<3 percentile or >97 percentile), and with positive results of the stool virus or bacterial PCR were excluded.
2.3. Questionnaires
The demographic and clinical characteristics were obtained from all the participating patients and their caregivers. All patients and/or their caregivers completed a detailed questionnaire about the birth history (gestational age, birth weight), weight, bowel habits, and a possible history of gastrointestinal problems or recent pathologic symptoms (diarrhea, vomit, poor oral intake).
2.4. Ethical considerations
We obtain written informed consent from all children guardians enrolled in this study. This study was approved by the Institutional Ethics Committee of CHA Bundang Medical Center, CHA University, Korea (approval No. BD2015‐008).
2.5. Sample collection
All participants received a plastic capped container and detailed instructions about the stool sample collection. The samples were brought directly to us or were delivered by mail in the same day or in the next day. Feces were immediately processed, homogenized, and stored in a −20°C freezer, when the analyses could be performed after the storage.
2.6. Fecal calprotectin measurement
A commercially available FEIA (Fluorescence Enzyme Immunoassay) (Green Cross Laboratories, Yongin‐si, Gyeonggi‐do, Korea) was used to measure quantitatively the concentration of FC. All collected stool samples were sent to Green Cross laboratories, and were measured for calprotectin concentrations. The supernatant of centrifuged solution with 0.2 g of stool was used to evaluate the calprotectin level by ImmunoCAP 250 (Phadia, Uppsala, Sweden). The lower detection limit was 11.5 μg/g, and higher detection limit was 2000 μg/g.
Concomitant virological, bacteriological, and multiplex PCR analyses were performed in all fecal samples. Positive result samples were excluded.
2.7. Statistical analysis
For calprotectin, with a non‐Gaussian distribution, the median is the preferred expression, but the arithmetic mean was computed for comparison with some literature reports.
SPSS statistical software package (IBM Corp., Armonk, NY, USA) was used for statistical analysis. In addition, GraphPad Prism (GraphPad Software, La Jolla, CA, USA) statistical software package was used for graphic calculation. Nonparametric Kruskal‐Wallis test was used to compare median and mean in different age groups. Associations among variable were assessed using the Spearman's rho and Dunnett's test.
3. Results
The study included a total of 234 samples from 234 children (54% boys) aged between 6 months and 4 years. The numbers of samples in different age groups are shown in Table 1. No difference was found in the FC concentration between the boys and the girls. The median FC concentration of all of the participants was 24.5 μg/g (range 12–1033 μg/g, mean 68.5 μg/g, SD 123.12 μg/g).
Table 1.
Characteristics of healthy children in different age groups
| Age | Group 1 7–12 months | Group 2 13–18 months | Group 3 19–24 months | Group 4 25–30 months | Group 5 31–36 months | Group 6 37–48 months | Total |
|---|---|---|---|---|---|---|---|
| No. patients (%) | 46 (19.6) | 39 (16.7) | 39 (16.7) | 49 (20.9) | 30 (12.8) | 31(13.2) | 234 (100) |
| % Male | 54 | 59 | 49 | 53 | 53 | 55 | 54 |
| Median of FC (95% CI) | |||||||
| Male | 103 (43.0‐135.0) | 29 (12.0‐72.0) | 12 (12.0‐91.0) | 12 (12.0‐40.0) | 12 (12.0‐22.5) | 12 (12.0‐12.0) | 18 (12.0‐40.0) |
| Female | 76 (42.5‐167.0) | 31 (14.0‐56.0) | 15 (12.0‐27.5) | 12 (12.0‐27.5) | 13.5 (12.0‐37.0) | 12 (12.0‐18.0) | 20.0 (13.0‐28.0) |
| Mean of FC | |||||||
| Male | 120.40 | 134.13 | 61.11 | 44.15 | 24.31 | 22.53 | 72.83 |
| Female | 176.29 | 55.31 | 37.10 | 38.30 | 22.43 | 23.50 | 63.45 |
FC, fecal calprotectin; CI, confidence interval.
The data were combined into six age groups divided at an interval of 6 months: 7–12, 13–18, 19–24, 25–30, 31–36, and 37–48 months (the children older than 37 months were merged into one group because no statistical difference was found between children aged 37–42 months and those aged 43–48 months). The collected data showed a significant difference between the means or medians in the various age groups. The cut‐off values were estimated by calculating the 95% percentile in each subclass.
The median and mean FC concentrations of all of the groups are shown in Table 2. A negative correlation trend was found between the age and the FC concentration (Spearman's rho −0.44, P=.01). The data are presented graphically in Figure 1. As the age increased, the mean, median, standard deviation, and the upper limit of 95% CI decreased.
Table 2.
FC levels (μg/kg) in different age groups of healthy children
| Age | Group 1 7–12 months | Group 2 13–18 months | Group 3 19–24 months | Group 4 25–30 months | Group 5 31–36 months | Group 6 37–48 months | Total |
|---|---|---|---|---|---|---|---|
| Median | 78.5 | 29 | 27 | 27 | 12.5 | 12 | 24.5 |
| 95% CI of median | |||||||
| Lower limit | 45 | 14 | 12 | 12 | 12 | 12 | 18 |
| Upper limit | 135 | 65 | 55 | 40 | 21 | 12 | 35 |
| Mean | 145.91 | 101.79 | 48.79 | 41.41 | 23.43 | 22.97 | 68.5 |
| Standard deviation | 185.29 | 176.12 | 68.46 | 46.12 | 20.00 | 26.63 | 123.12 |
| Standard error | 27.32 | 23.20 | 10.96 | 6.59 | 3.65 | 4.78 | 8.05 |
| Minimum | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Maximum | 1033 | 769 | 301 | 200 | 87 | 106 | 1033 |
FC, fecal calprotectin; CI, confidence interval.
Figure 1.
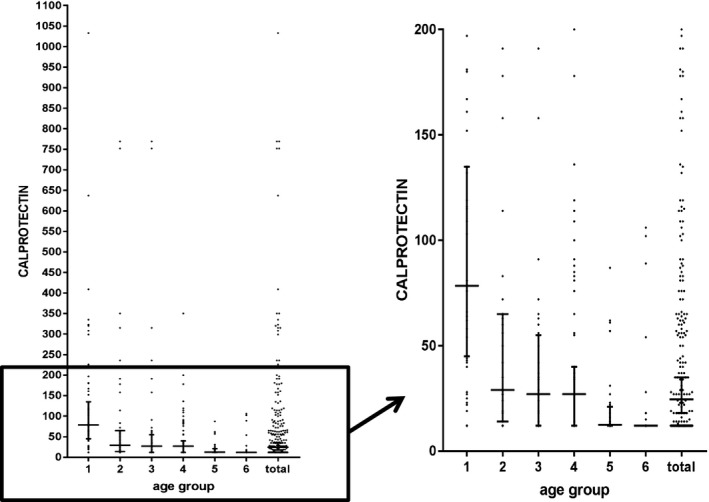
Diagram of FC levels in healthy children (short horizontal lines indicate 95% percentiles, long horizontal lines indicate medians)
Two of the FC results (1331 μg/g at the age of 35 months and 715 μg/g at the age of 29 months) were excluded because the values were significantly different from the each group (P<.05).
4. Discussion
In this study, we found higher FC levels in healthy children aged <4 years than in adults. In addition, the FC levels showed a significant negative correlation with age. Therefore, it may be postulated that FC levels are associated with the ongoing developmental processes of the digestive tract.
Originally, the fetal gastrointestinal (GI) tract is sterile. The establishment of the gut microbiota is immediately initiated at birth and characterized by a dynamic succession of bacterial populations until a homeostatic adult‐like microbiota is established by the age of 2–3 years.11 Thus, these high basal calprotectin levels could be due to a higher intestinal permeability, the establishment of gut flora, and a response to alimentary antigens, as well as to the colonization of the gut by commensal microbes, which help to prevent enteric pathogen infections and block the interactions between the pathogens and the host cells. FC concentrations decrease with age. This reduction may indicate a maturation process, including decreased intestinal permeability of the intestinal mucosa. The upper limit of 95% CI of median value was 135 μg/g between 7 and 12 months, 65 μg/g between 13 and 18 months, 55 μg/g between 19 and 24 months, 40 μg/g between 25 and 30 months, 21 μg/g between 31 and 36 months, and 12 μg/g between 37 and 48 months. Our results show that at approximately the age of 2 years the upper limit of 95% CI of median values of the children FC levels reach the adult's normal range (below 50 μg/g). This can be considered the point of the complete intestinal mucosa maturation.
When we started this study, we planned to collect the FC values beginning with birth, but the FC values under the age of 6 months varied considerably (11.5–1330.6 μg/g) and it was difficult to establish a cut‐off value. In other study performed by us regarding the FC level in infants aged between 0 and 6 months, we found that FC level in this age group varied considerably and was affected by the type of feeding or the delivery mode (Y. M. Lee, unpublished data). FC values were significantly higher in breastfed children and those born by normal spontaneous vaginal delivery (NSVD). Conversely, at the age of 7–12 months, the variation in FC levels decreased and was not affected by the feeding type or the delivery mode. Therefore, it is mandatory for the clinical application of the FC level to consider the possible factors that can influence the FC level in children aged <6 months, such as the delivery mode or the feeding type.
Some studies indicated regional and racial differences in pediatric FC levels.12, 13, 14, 15, 16, 17, 18, 19, 20 Furthermore, several studies from China found differences in FC levels even in the same country.12, 21, 22 Although the age variation makes direct comparisons among different sites difficult to interpret, our study showed lower levels of the FC values compared to the most recently published data by Zhu et al.21 on the Chinese children FC levels. They measured the FC levels in healthy children aged 1–4 years from Shanghai. Table 3 compares the FC levels reported in our study with those found in Shanghai children. Our study shows lower FC levels than in Shanghai in each age group. In addition, the FC levels were influenced by the gut inflammation or microbiota, which is determined by the public hygiene. Based on the FC level gap between different countries and different cities in same country, we can conclude that the characteristics of the area, such as social income, habits, or public health value, determine the status of sanitation and affect the FC level.
Table 3.
FC levels (μg/kg) compared to other region
| Age | N (%) | Median FC (5th–95th percentile) | Age | N (%) | Median FC (5th–95th percentile) |
|---|---|---|---|---|---|
| 6–12 months | 46 (19.6) | 78.5 (45‐135) | |||
| 1–2 years | 119 (43.43) | 96.14 (19.67‐447.73) | |||
| 13–18 months | 39 (16.7) | 29 (14‐65) | |||
| 19–24 months | 39 (16.7) | 27 (12‐55) | |||
| 2–3 years | 72 (26.28) | 81.48 (16.61‐368.04) | 25–30 months | 49 (20.9) | 27 (12‐40) |
| 31–36 months | 30 (12.8) | 12.5 (12‐21) | |||
| 3–4 years | 83 (30.29) | 65.36 (9.78‐39.33) | 37–48 months | 31 (13.2) | 12 (12‐12) |
| Total | 274 | 83.19 | 234 | 68.5 |
FC, fecal calprotectin.
In addition to GI diseases, the FC level was recently used as a diagnostic marker for various atopic and allergic diseases in children.6, 7, 8, 9, 10 In our study, three children with high FC values had allergic diseases, one 34‐month‐old child with high FC value was diagnosed with cow's milk protein allergy, and other 24‐month‐old and 29‐month‐old children without food allergy had a history of atopic dermatitis. This might suggest a correlation between the allergic diseases and the FC level and the necessity of the large population study about the low‐grade inflammation of the gut or the increase in gut permeability induced by the allergy.
Although the FC level can suggest the inflammation of the gut, the high level of calprotectin cannot be considered an indicator to differentiate the symptoms of chronic inflammatory bowel diseases from the acute bowel infections. Thus, this fact should be considered in interpreting the results.
In addition, numerous ongoing studies investigate the relationship of the FC levels with various bowel diseases, such as acute appendicitis, Henoch‐Schönlein purpura, and colonic polyps.23, 24, 25 Several studies showed that even neonates who suffered from necrotizing enterocolitis had early elevation in FC levels.26 Based on these results, further studies should investigate the FC levels in different diseases.
This study has several strengths. This is the first study reporting the FC levels measured in a large Korean cohort of healthy infants and small children aged <4 years. The results are highly comparable to previous findings and clearly show that healthy, younger children have higher FC concentration than older children. The differences in FC levels in these studies may be influenced by genetic and environmental factors. As the age increases, the median, mean, variation, and the cut‐off values decrease and show a significant statistical difference between the groups. In addition, to our best knowledge this is the first study to suggest specific cut‐off levels in specific age groups, <4 years.
However, our study has several limitations. First, the stool samples were collected by mail and no information regarding the place in which the stool was collected (from stool, toilet, etc.) was available. The diaper could absorb the water from the stool or toilet water might contaminate the stool. Olafsdottir et al.14 reported that the stool from diaper increases the FC concentration by up to 30% by decreasing the water from the stool. The direct collection at stool emission might be more practical. Second, our study is a cross‐sectional study and we did not further investigate the children with elevated FC concentrations to evaluate whether these concentrations normalized over time or developed the symptoms of bowel diseases.
5. Conclusions
The FC levels of children aged 6–48 months show a downward trend with age and are higher than the normal levels observed in healthy adults and older children. In our study, the FC levels reached the 50 μg/g value around the age of 2 years. The FC values found in this study could be useful in future studies to investigate different gastrointestinal disorders in younger children.
Acknowledgments
We are grateful to Green Cross Laboratory and Phadia Korea for the donation of the calprotectin assays. We also thank attendants and parents for their participation and kind assistance.
Song, J. Y. , Lee, Y. M. , Choi, Y. J. and Jeong, S. J. Fecal calprotectin level in healthy children aged less than 4 years in South Korea. Journal of Clinical Laboratory Analysis. 2017;31:e22113 10.1002/jcla.22113
References
- 1. Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron‐sequestering host‐defense protein. Nat Chem Biol. 2015;11:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johne B, Fagerhol M, Lyberg T, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Limburg PJ, Ahlquist DA, Sandborn WJ, et al. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–2837. [DOI] [PubMed] [Google Scholar]
- 4. Alibrahim B, Aljasser MI, Salh B. Fecal calprotectin use in inflammatory bowel disease and beyond: a mini‐review. Can J Gastroenterol Hepatol. 2015;29:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fagerberg UL, Lööf L, Lindholm J, Hansson L‐O, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414–420. [DOI] [PubMed] [Google Scholar]
- 6. TrilloBelizon C, Ortega Páez E, Medina Claros AF, et al. Faecal calprotectin as an aid to the diagnosis of non‐IgE mediated cow's milk protein allergy. An Pediatr (Barc). 2016;84:318–323. [DOI] [PubMed] [Google Scholar]
- 7. Orivuori L, Mustonen K, de Goffau MC, et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin Exp Allergy. 2015;45:928–939. [DOI] [PubMed] [Google Scholar]
- 8. Merras‐Salmio L, Kolho KL, Pelkonen AS, Kuitunen M, Makela MJ, Savilahti E. Markers of gut mucosal inflammation and cow's milk specific immunoglobulins in non‐IgE cow's milk allergy. Clin Transl Allergy. 2015;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beşerz ÖF, Sancak S, Erkan T, Kutlu T, Çokuğraş H, Çokuğraş FÇ. Can fecal calprotectin level be used as a markers of inflammation in the diagnosis and follow‐up of cow's milk protein allergy? Allergy Asthma Immunol Res. 2014;6:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldassarre ME, Fanelli M, Lasorella ML, et al. Fecal calprotectin (FC) in newborns: is it a predictive marker of gastrointestinal and/or allergic disease? Immunopharmacol Immunotoxicol. 2011;33:220–223. [DOI] [PubMed] [Google Scholar]
- 11. Avershina E, Storrø O, Øien T, et al. Bifidobacterial succession and correlation networks in a large unselected cohort of mothers and their children. Appl Environ Microbiol. 2013;79:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu JR, Sheng XY, Hu YQ, et al. Fecal calprotectin levels are higher in rural than in urban Chinese infants and negatively associated with growth. BMC Pediatr. 2012;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177. [DOI] [PubMed] [Google Scholar]
- 14. Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Actapaediatrica. 2002;91:45–50. [DOI] [PubMed] [Google Scholar]
- 15. Rugtveit J, Fagerhol MK. Age‐dependent variations in fecal calprotectin concentrations in children. J Pediatr Gastroenterol Nutr. 2002;34:323. [DOI] [PubMed] [Google Scholar]
- 16. Fagerberg UL, Lööf L, Merzoug RD, Hansson L‐O, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. 2003;37:468–472. [DOI] [PubMed] [Google Scholar]
- 17. Canani RB, Rapacciuolo L, Romano M, et al. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liv Dis. 2004;36:467–470. [DOI] [PubMed] [Google Scholar]
- 18. Bremner A, Roked S, Robinson R, Phillips I, Beattie M. Faecal calprotectin in children with chronic gastrointestinal symptoms. Acta Paediatr. 2005;94:1855–1858. [DOI] [PubMed] [Google Scholar]
- 19. Hestvik E, Tumwine JK, Tylleskar T, et al. Faecal calprotectin concentrations in apparently healthy children aged 0‐12 years in urban Kampala, Uganda: a community‐based survey. BMC Pediatr. 2011;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oord T, Hornung N. Fecal calprotectin in healthy children. ScandClin Lab Invest. 2014;74:254–258. [DOI] [PubMed] [Google Scholar]
- 21. Zhu Q, Li F, Wang J, Shen L, Sheng X. Fecal calprotectin in healthy children aged 1‐4 years. PLoS ONE. 2016;11:e0150725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li F, Ma J, Geng S, Wang J, Ren F, Sheng X. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum Dev. 2014;90:471–475. [DOI] [PubMed] [Google Scholar]
- 23. Ambe PC, Gödde D, Bönicke L, Papadakis M, Störkel S, Zirngibl H. Calprotectin could be a potential biomarker for acute appendicitis. J Transl Med. 2016;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanik A, Baran M, Ince FD, et al. Faecal calprotectin levels in children with Henoch–Schönlein purpura: is this a new marker for gastrointestinal involvement? Eur J Gastroenterol Hepatol. 2015;27:254–258. [DOI] [PubMed] [Google Scholar]
- 25. Pezzilli R, Barassi A, Labate AM, et al. Fecal calprotectin levels in patients with colonic polyposis. Dig Dis Sci. 2008;53:47–51. [DOI] [PubMed] [Google Scholar]
- 26. Pergialiotis V, Konstantopoulos P, Karampetsou N, et al. Calprotectin levels in necrotizing enterocolitis: a systematic review of the literature. Inflamm Res. 2016;65:1–6. [DOI] [PubMed] [Google Scholar]


