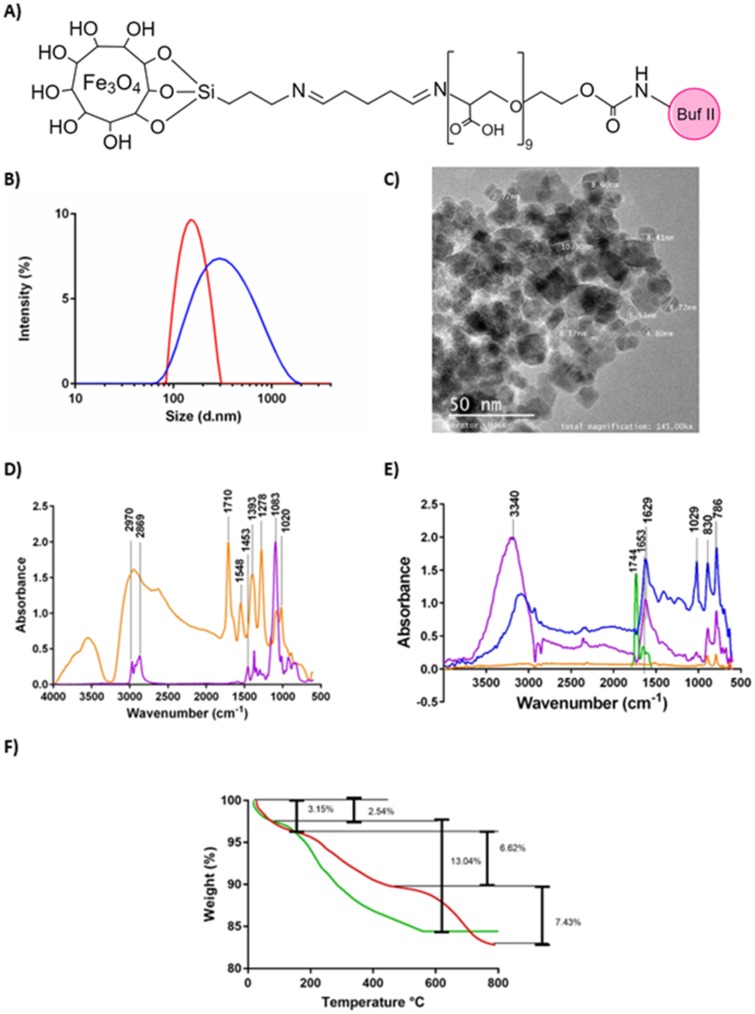
Figure 1.
(A) Schematic of the chemical structure of the nanobioconjugate. (B) DLS histogram for the size distribution of magnetite nanoparticles (red) and BUFII-PEA-Magnetite nanobioconjugates (blue). (C) TEM micrograph of the BUFII-PEA-Magnetite nanobioconjugates with the approximate diameters of individual particles (D) FTIR spectra of oxidized PEA (orange), and PEA (purple). (E) FTIR spectra of bare magnetite (orange), PEA-coated magnetite (blue), BUF-II (green) and BUFII-PEA-Magnetite nanobioconjugates (purple). (F) TGA thermograms of magnetite (green) and BUFII-PEA-Magnetite nanobioconjugates (red). The first weight loss steps (2.54 and 3.15%) represent the dehydration of the samples. Second weight loss steps (6.62% and 13.04%) correspond to physically adsorbed organic solvents. The final weight loss step (7.43%) is attributed to the detachment of BUF-II from the nanoparticle’s surface.