Abstract
Aim
To review the published literature on the treatment of aneurysmal bone cysts (ABCs).
Method
A systematic review of the English literature to April 2019 for all articles, with a minimum of three patients and 2-year follow-up, reporting on the treatment of spinal ABCs. The various treatment options were compared for the rates of recurrence, complications and mortality.
Results
Twenty-one articles and 272 patients (mean age 16.9 years, range 3–67) were included in this review. The overall recurrence rate for ABCs following all treatments is 12.8%. This is highest in those lesions described as being treated with isolated surgiflo injection into the lesion (100%), decompression/laminectomy (42.3%), partial excision/resection (35.7%) and curettage alone (25.0%). Radiotherapy alone or in conjunction with operative intervention offers excellent cure rates. Adjuncts to operative intervention, including cryotherapy or phenol reduce the recurrence rates, whereas embolization does not. The most common complications are persistent neurological deficits, spinal deformity, and continued pain. The overall mortality rates are low (1.5%). The reoperation rates are higher in surgical than non-surgical treatments and most are performed for progressive deformity.
Discussion
ABCs are highly radiosensitive. However, with the unknown longer-term risk of radiotherapy, surgical treatments, ideally with complete resection, and the use of adjunctive therapies such as cryotherapy or phenol, offer the best chance of cure. SAE is a useful adjunct to reduce intraoperative bleeding, but this study suggests that it only modestly improves recurrence rates. Newer techniques including bisphosphonate and doxycycline administration offer potential benefits, but their efficacy requires further investigation.
Keywords: spine, tumour, review, aneurysmal bone cyst, ABC
Introduction
Aneurysmal bone cysts (ABCs) are benign skeletal lesions that account for 1% of primary bone tumours and predominantly affect those in the first 20 years of life.1 The pathophysiology of ABCs is debated but most authors propose that they develop as a result of intercellular oedema caused by the primary lesion expanding the surrounding loose stroma and permitting rupture of vessels into the microcysts under haemodynamic pressure, thereby causing a blood-filled cyst.1 They most commonly affect the long bones, but up to 20% occur in the spine, predominantly the posterior elements.2–7 These cysts tend to present with pain and swelling in the region of the lesion and therefore spinal ABCs typically present with pain, deformity and neurological dysfunction.4–10
Definitive diagnosis is obtained from radiological and histological findings.5–7,11 On x-ray and CT imaging they appear as expansile osteolytic lesions with thin sclerotic margins and fluid-fluid levels. On MRI imaging the cyst is seen to be separated by septae of connective tissue and bone and the fluid-fluid levels are accentuated. There is also often a rim of peripheral enhancement.5,12–14 On bone scan the peripheral rim of increased uptake is seen as a “doughnut sign” and on angiography the cyst is seen to be poorly vascular. Macroscopically, these lesions appear as spongy haemorrhagic masses covered by a thin shell of reactive bone. Histologically, they appear as multiple large blood-filled spaces separated by fibrous or osseous septae and lined by fibroblasts and histiocytes. Osteoclastic giant cells and mitotic figures are typically present, but malignant osteoid and atypia are not.
The behaviour of these lesions varies; however, most behave aggressively with progressive expansion and bone destruction. Thus, traditionally, the treatment of ABCs has been surgical. In the spine, en bloc or marginal resection was preferred, but not always achievable because of the high risk of iatrogenic neurological injury.1,8,12,15 Curettage was, therefore, the most common method of surgical treatment, but was seen to carry a high risk of recurrence, usually within 6 months of the index procedure.2,8,15 Radiation therapy has been proposed as a primary treatment or as an adjuvant; however, caution exists due to longer-term malignant transformation and the proximity to neurological structures.15,16 More recent advances in targeted radiotherapy, such as stereotactic radiotherapy, have permitted a more localised field of radiation and therefore a lower risk of neurological injury, but still carries a radiation risk.15
Selective arterial embolisation (SAE) is another technique increasing in popularity.1,3,17 This often requires multiple treatments for success, and concerns exist about the remaining blood supply to the surrounding tissues and repeat radiological exposure.3,18–20 Other emerging treatments for ABCs include bisphosphonates, percutaneous doxycycline and sclerotherapy, because they are felt to carry less morbidity and achieve a cure.1,8,12,13,21,22 This review was conducted to assess the efficacy of spinal ABC treatments reported in the literature.
Method
Search Criteria
This analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology guidelines. The search strategy aimed to find published studies reporting on the management of spinal aneurysmal bone cysts. A three-step search strategy was utilised.
Firstly, a comprehensive search of all publications in the electronic databases up to April 2019 was independently conducted by two investigators (JP and DK) using Medline, Embase, Google Scholar and the Cochrane Library. The MeSH terms (Spine and Aneurysmal Bone Cyst) and keywords (Spinal, ABC) connected by the Boolean operators “AND” and “OR” were used to identify all possible studies. The identified articles were then reviewed for keywords contained in the title, abstract or the index terms used to describe the study subject. Secondly, a search using all identified keywords and index terms was undertaken across all of the included databases. Finally, a manual search was conducted of the reference lists of identified articles, relevant reviews and prominent textbooks for additional studies.
Inclusion Criteria
All studies had to meet the following inclusion criteria: 1. Studies reporting on the outcomes of management of ABCs (regardless of treatment modality); 2. Randomised control trials, non-randomised control trials, case series; 3. Minimum follow-up of 2 years; 4. Studies published in English. 5. Three or more patients in case-series.
Exclusion Criteria
Studies were excluded if they had any of the following characteristics: 1. Duplicate or overlapping data; 2. Unclear treatment, 3. Ambiguous outcome reporting.
Article Assessment
All papers selected for inclusion were assessed independently by the reviewers (JP and DK) for methodological validity prior to inclusion in the review using standardised critical appraisal instruments from the Joanna Briggs Institute Meta Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI). Bias was assessed by the reviewers (JP and DK) using the Robins-I assessment tool.
Outcomes Of Interest
The primary outcome measure for this literature review was a local recurrence. Secondary outcome measures were; complication rates and profile, reoperation rates and mortality.
Data Extraction
The reviewers (JP and DK) independently extracted quantitative data from all eligible studies using a standardised data recording spreadsheet. The data of interest included the following categories:
Study characteristics including study type, year of publication, cohort size, age, and follow-up duration.
Treatment information including
Surgical: (en bloc or curettage ± local adjunct (cement, autologous bone graft, bone graft substitute, other)) and adjuncts to surgery (radionuclide ablation, radiotherapy, sclerotherapy, cryotherapy, selective arterial embolisation, bisphosphonates, denosumab).
Non-surgical: radionuclide ablation, radiotherapy, sclerotherapy, cryotherapy, selective arterial embolisation, bisphosphonates, denosumab.
Numbers of patients with the primary outcome.
Numbers of patients with each secondary outcome.
Statistical Analysis
Baseline demographics were reported as a mean and range. Due to the vast heterogeneity of the results and lack of direct comparison between treatment groups, no statistical method would offer comparative validity and therefore the outcome measures are presented as raw figures.
Results
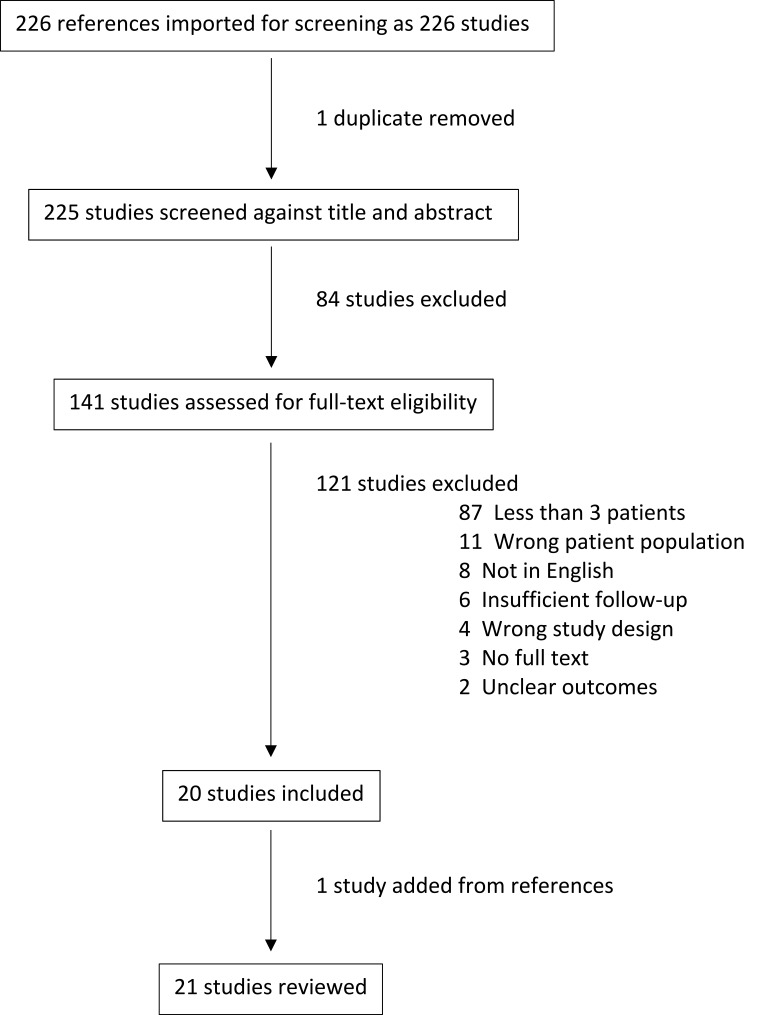
Our initial search returned 225 papers for screening related to ABCs. Of these, a large proportion were either not related to the spine, or single case reports. This led to the selection of 20 papers for data extraction from our search. One paper was added from the review of article references resulting in 21 papers included in this review (Figure 1).
Figure 1.
PRISMA chart.
The included article characteristics are provided in Table 1. Appendix 1 displays the Robins-I assessment for the bias of the included studies. A total of 281 patients were identified in these articles; however, nine patients failed to reach a minimum of 24 months of follow up and were therefore excluded from further review.
Table 1.
Articles And Patients Included In This Study
| First Author | Year | Location | Patients Includeda |
|---|---|---|---|
| Ameli23 | 1985 | Italy | 9 |
| Amendola17 | 2013 | Italy | 7 |
| Boriani24 | 2001 | Italy | 41 |
| Capanna25 | 1985 | Italy | 22 |
| Chao16 | 2014 | China | 10 |
| Daszkiewicz14 | 2004 | Poland | 8 |
| De Kleuver9 | 1998 | Netherlands | 24 |
| Feigenburg15 | 2001 | USA | 5 |
| Garg2 | 2005 | USA | 10 |
| Geffroy26 | 2012 | France | 5 |
| Kieser8 | 2018 | France | 6 |
| Mesfin7 | 2012 | USA | 8 |
| Novais27 | 2011 | USA | 6 |
| Ozaki28 | 1998 | Germany | 13 |
| Papagelopoulos34 | 1998 | USA | 40 |
| Rossi1 | 2010 | Italy | 5 |
| Sebaaly13 | 2015 | France | 4 |
| Shiels12 | 2013 | USA | 5 |
| Terzi3 | 2017 | Italy | 15 |
| Zenenos11 | 2012 | USA | 11 |
| Zileli6 | 2013 | Turkey | 18 |
Note: aPatients from these studies without 24 months of follow-up were omitted from the review.
Table 2 displays the included patient baseline characteristics.
Table 2.
Patient Presentation Characteristics
| Total Patients | 272 |
|---|---|
| Agea | 16.9 years (3–67) |
| Sex ratio (M:F) | 1:0.98 |
| Follow upa | 75.4 months (24–257) |
| Vertebral levelb | |
| Cervical | 85 |
| Thoracic | 74 |
| Lumbar | 85 |
| Sacral | 33 |
| Presentation | |
| Pain | 249 |
| Neurology | 81 |
| Enneking stage (n = 62) | |
| 1 | 2 |
| 2 | 16 |
| 3 | 44 |
| Frankel grade (n = 58) | |
| E | 53 |
| D | 3 |
| C | 0 |
| B | 1 |
| A | 1 |
Note: aMean values. bSome lesions affected more than one vertebral level.
The recurrence rate for each treatment modality is outlined in Table 3. The overall recurrence rate for ABCs following all treatments is 12.8%. This is highest in those lesions described as being treated with isolated surgiflo injection into the lesion (100%), decompression/laminectomy (42.3%), partial excision/resection (35.7%) and curettage alone (25.0%). A total of 85 (40%) patients undergoing surgical management had spinal instrumentation (either stabilisation or fusion) at the time of their procedure.
Table 3.
Summary Of Reviewed Treatments
| Primary Treatment | Patients | Recurrence | Recurrence As % |
|---|---|---|---|
| Surgical | 213 | 29 | 13.6 |
| Complete excision/resection | 97 | 8 | 8.2 |
| Complete excision/resection + SAE | 13 | 1 | 7.7 |
| Complete excision/resection + RT | 9 | 0 | 0 |
| Partial excision/resection | 14 | 5 | 35.7 |
| Partial excision/resection + RT | 13 | 1 | 7.7 |
| Curettage only | 40 | 10 | 25 |
| Curettage + RT | 12 | 0 | 0 |
| Curettage + SAE | 6 | 1 | 16.7 |
| Curettage + Phenol | 1 | 0 | 0 |
| Curettage + Cryotherapy | 1 | 0 | 0 |
| Laminectomy/decompression | 7 | 3 | 42.3 |
| Non-operative | 59 | 6 | 10.2 |
| SAE | 27 | 4 | 14.8 |
| Radiotherapy | 19 | 0 | 0 |
| Bisphosphonate | 6 | 0 | 0 |
| Doxycycline | 5 | 0 | 0 |
| Surgiflo | 2 | 2 | 100 |
Abbreviations: SAE, selective arterial embolisation; RT, radiation therapy.
SAE was found to commonly require multiple treatments (average 3.4, range 1–14) and to have a recurrence rate of 14.8%. Small studies of non-surgical treatments such as bisphosphonate (6 patients, average 2.3 treatments) or doxycycline (5 patients, average 6.6 treatments) injections have shown favourable outcomes with no recurrence.
The complication profile of each treatment modality was poorly reported. Table 4 displays the complication profile for the various treatments. The reoperation rate for surgical treatments was 5.2% with most performed for progressive deformity. The operation rate for initially non-operative treatment of ABCs was 1.7%, but this was again poorly reported. About 6.1% of patients undergoing operative intervention had significant bleeding requiring transfusion and this was more common in procedures that breached the cyst rather than complete excision.
Table 4.
Complication Profile For Each Treatment Modality
| Treatment | Number | Reoperation | Bleeding/Transfusion | Deformity | Persistent Neurology | Persistent Pain | Infection | Other | Death | |
|---|---|---|---|---|---|---|---|---|---|---|
| Surgicala | 213 | 11 | 13 | 12 | 13 | 5 | 2 | 7 | 5 | |
| Complete excision/resection | 97 | 4 (fusion) | 1 | 3 | 1 | 1 | Dural tear | |||
| Complete excision/resection + SAE | 13 | 1 | 1 | |||||||
| Complete excision/resection + RT | 9 | Radiation induced hypothyroid | ||||||||
| Partial excision/resection | 14 | 5 (fusion) | 3 | 5 | 4 | Dural tear | ||||
| Partial excision/resection + RT | 13 | 1 (fusion) | ||||||||
| Curettage only | 40 | 1 (re-excision) | 3 | 2 | 3 | Pseudarthrosis | ||||
| Curettage + RT | 12 | 2 | ||||||||
| Curettage + SAE | 6 | 2 | 1 | 2 | 1 | |||||
| Curettage + Phenol | 1 | |||||||||
| Curettage + Cryotherapy | 1 | 1 | 1 | |||||||
| Laminectomy/decompression | 7 | 1 | 1 | |||||||
| Non-operative | 59 | 1 | 1 | 1 | 1 | |||||
| SAE | 27 | 1 (fusion) | 1 | |||||||
| Radiotherapy | 19 | 1 | 1 | |||||||
| Bisphosphonate | 6 | |||||||||
| Doxycycline | 5 | |||||||||
| Surgiflo | 2 | |||||||||
Note: aSome articles report an overall complication profile rather than the specific complications for each treatment modality.
Table 5 summates the overall complications and shows that the most common complications are persistent neurological deficits (6.6%), spinal deformity (5.5%) and continued pain (2.6%). The overall mortality rates are low (1.5%).
Table 5.
Reported Complications
| Complication | Number Of Patientsa |
|---|---|
| Neurologicalb | 18 |
| Deformity | 15 |
| Pain | 7 |
| Death | 4 |
| Chronic infection | 2 |
Notes: aSome patients experienced more than one complication. bWeakness or sensory loss.
Discussion
This review documented 272 reported cases of spinal ABCs. Across all treatment methods, there were 35 patients who had recurrences, at an overall rate of 12.8%, which is in keeping with others quoted in the literature.9 Rates of recurrence differed between surgical treatment methods and were higher when incomplete removal of the lesion occurred. Illustrating this is the recurrence rates of decompression/laminectomy (42.3%), partial excision (35.7%) and curettage (25%) when compared to complete excision/resection (8.2%). The results of this study suggest that the success of a surgical intervention, such as curettage, is enhanced with adjuncts such as radiotherapy, phenol and cryotherapy (0% recurrence rate when combined with surgical curettage). In contrast, embolisation may enhance the surgical ease with reduced bleeding, but in itself only appears to marginally reduce the recurrence rates of surgically treated ABCs (16.7% recurrence rate when combined with surgical curettage).
The results of this study also demonstrate less significant bleeding if the ABC cavity is not breached, but that persistent pain, neurology and deformity requiring reoperation are common in surgically treated lesions. In contrast, radiotherapy appears to offer excellent cure rates, although the longer-term effects, including malignant transformation, remain unexplored in this study. Because of this uncertainty, if radiotherapy is to be used, the radiation dose should be kept as low as possible and use modern techniques with megavoltage radiation in an attempt to reduce the risk of malignant transformation.29,30 Furthermore, the studies included in this review report external beam radiotherapy; however, alternative options such as percutaneous radionuclide ablation have shown success.31 In contrast to radiotherapy, isolated SAE requires multiple treatments (average 3.4) and has a high recurrence rate (14.8%). Small reports on the use of bisphosphonate or doxycycline injections offer promise with no recurrence reported, but remain unvalidated by larger independent studies.
Our search criteria excluded studies with less than 2-year follow-up and a minimum of three patients with a specific focus on spinal ABCs in an attempt to limit reporting bias. However, there is a growing interest in novel biological approaches to treat ABCs. Specific genetic translocations have been identified in ABCs which offer potential targets for gene therapy.32 Furthermore, targeted molecular therapy such as denosumab has been reported with encouraging results in spinal ABCs, but the number of patients treated remain limited.33
This study has a number of limitations. Firstly, the included studies include patients from as far back as 1910 and therefore follow-up methods, radiological techniques and treatments are not uniform.34 Secondly, none of the included articles directly compares treatment methods in a randomised, blinded controlled fashion. Thirdly, all articles have a significant bias as evidenced by the Robins-I assessment and the selection criteria for lesions that are amenable to non-operative treatment is not equivocal to those for operative intervention. Lastly, the exact description of treatments, including surgical techniques and adjuncts is poorly defined. For example, a few articles describe their surgical treatment as laminectomy and do not further extrapolate. However, these lesions typically affect the posterior elements and a laminectomy may permit a complete resection, partial resection or an isolated decompression. Similarly, the precise radiation protocol is poorly defined in most articles.
Despite these limitations, this study would suggest that ABCs are highly radiosensitive. However, with the unknown longer-term risk of radiotherapy, this should be reserved for persistent or recurrent ABCs rather than the primary treatment. Primary treatment should be surgical, ideally with complete resection, and the use of adjunctive therapies such as cryotherapy or phenol, which offer the best chance of cure. SAE is a useful adjunct to reduce intraoperative bleeding, but this study suggests that it only modestly improves recurrence rates. Newer techniques including bisphosphonate and doxycycline administration offer potential benefit, but their efficacy requires further investigation.
Acknowledgment
The authors acknowledge Glynny Kieser for her editorial input.
Disclosure
Dr Ibrahim Obeid reports grants and personal fees from Depuy, personal fees from Medtronic, royalties from Clariance, Spineart, and Alphatec, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol. 2010;39(2):161–167. [DOI] [PubMed] [Google Scholar]
- 2.Garg S, Mehta S, Dormans JP. Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J Pediatr Orthop. 2005;25(3):387–392. [DOI] [PubMed] [Google Scholar]
- 3.Terzi S, Gasbarrini A, Fuiano M, et al. Efficacy and safety of selective arterial embolization in the treatment of aneurysmal bone Cyst of the mobile spine. Spine (Phila Pa 1976). 2017;42(15):1130–1138. [DOI] [PubMed] [Google Scholar]
- 4.Aljoghaiman MS, Alhamad SM, Homan MA, Harfouch BF. Aneurysmal bone cyst of the spine: report of four cases and review of the literature. Interdiscip Neurosurg. 2019;16:18–21. [Google Scholar]
- 5.Riahi H, Mechri M, Barsaoui M, Bouaziz M, Vanhoenacker F, Ladeb M. Imaging of benign tumors of the osseous spine. J Belgian Soc Radiol. 2018;102(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zileli M, Isik HS, Ogut FE, Is M, Cagli S, Calli C. Aneurysmal bone cysts of the spine. Eur Spine J. 2013;22(3):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesfin A, McCarthy EF, Kebaish KM. Surgical treatment of aneurysmal bone cysts of the spine. Iowa Orthop J. 2012;32(1):40–45. [PMC free article] [PubMed] [Google Scholar]
- 8.Kieser DC, Mazas S, Cawley DT, et al. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J. 2018;27(4):851–858. [DOI] [PubMed] [Google Scholar]
- 9.De Kleuver M, Van Der Heul RO, Veraart BEEMJ. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop Part B. 1998;7(4):286–292. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe HL, Lichtenstein L. Solitary unicaramel bone cyst. Arch Surg. 1942;44(6):1004–1025. [Google Scholar]
- 11.Zenonos G, Jamil O, Governale LS, Jernigan S, Hedequist D, Proctor MR. Surgical treatment for primary spinal aneurysmal bone cysts: experience from Children’s Hospital Boston. J Neurosurg Pediatr. 2012;9(3):305–315. [DOI] [PubMed] [Google Scholar]
- 12.Shiels WE, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report tumor. Clin Orthop Relat Res. 2013;471(8):2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebaaly A, Ghostine B, Kreichati G, et al. Aneurysmal bone cyst of the cervical spine in children: a review and a focus on available treatment options. J Pediatr Orthop. 2015;35(7):693–702. [DOI] [PubMed] [Google Scholar]
- 14.Daszkiewicz P, Roszkowski M, Grajkowska W. Aneurysmal bone cyst of skull and vertebrae in children. Analysis of own material and review of the literature. Folia Neuropathol. 2004;42(1):25–30. [PubMed] [Google Scholar]
- 15.Feigenberg SJ, Marcus RB, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys. 2001;49(5):1243–1247. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Liu XG, Jiang L, et al. Treatments for primary aneurysmal bone cysts of the cervical spine: experience of 14 cases. Chin Med J (Engl). 2014;127(23):4082–4086. doi: 10.3760/cma.j.issn.0366-6999.20132099 [DOI] [PubMed] [Google Scholar]
- 17.Amendola L, Simonetti L, Simoes CE, Bandiera S, De Iure F, Boriani S. Aneurysmal bone cyst of the mobile spine: the therapeutic role of embolization. Eur Spine J. 2013;22(3):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahesh M. Imaging and therapeutic technology. The AAPM/RSNA physics tutorial for residents fluoroscopy: patient radiation exposure issues 1. RadioGraphics. 2001;21. [DOI] [PubMed] [Google Scholar]
- 19.Tse G, Spies JB. Radiation exposure and uterine artery embolization: current risks and risk reduction. Tech Vasc Interv Radiol. 2010;13(3):148–153. [DOI] [PubMed] [Google Scholar]
- 20.Glazier JJ, Dixon SR. Skin injury following prolonged fluoroscopy: early and late appearances. QJM. 2012;105(6):571–573. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi S, Varshney MK, Trikha V, Khan SA, Choudhury B, Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. J Bone Joint Surg Br. 2006;88–B(9):1212–1216. doi: 10.1302/0301-620X.88B9.17829 [DOI] [PubMed] [Google Scholar]
- 22.Brosjö O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84(5):502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameli NO, Abbassioun K, Saleh H, Eslamdoost A. Aneurysmal bone cysts of the spine. J Neurosurg. 1985;63:685–690. [DOI] [PubMed] [Google Scholar]
- 24.Boriani S, De Lure F, Campanacci L, et al. Aneurysmal bone cysts of the mobile spine. Spine. 2001;26(1):27–35. [DOI] [PubMed] [Google Scholar]
- 25.Capanna R, Albisinni U, Picci P, Calderoni P, Campanacci M, Springfield DS. Aneurysmal bone cyst of the spine. J Bone Joint Surg. 1985;67(4):527–531. [PubMed] [Google Scholar]
- 26.Geffroy L, Hamel O, Odri GA, et al. Treatment of an aneurysmal bone cyst of the lumbar spine in children and teenagers, about five cases. J Pediatr Orthop. 2012;21:269–275. [DOI] [PubMed] [Google Scholar]
- 27.Novais EN, Rose PS, Yaszemski MJ, Sim FH. Aneurysmal bone cyst of the cervical spine in children. J Bone Joint Surg Am. 2011;93:1534–1543. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki T, Halm H, Hillmann A, Blasius S, Winkelmann W. Aneurysmal bone cysts of the spine. Arch Orthop Trauma Surg. 1999;119:159–162. [DOI] [PubMed] [Google Scholar]
- 29.Zhu S, Hitchcock KE, Mendenhall WM. Radiation therapy for aneurysmal bone cysts. Am J Clin Oncol. 2015;40(6):621–624. [DOI] [PubMed] [Google Scholar]
- 30.Elsayad K, Kriz J, Seegenschmiedt H, et al. Radiotherapy for aneurysmal bone cysts: a rare indication. Strahlenther Onkol. 2017;193(4):332–340. [DOI] [PubMed] [Google Scholar]
- 31.Bush CH, Adler Z, Drane WE, Tamurian R, Scarborough MT, Gibbs CP. Percutaneous radionuclide ablation of axial aneurysmal bone cysts. Am J Roentgenol. 2010;194(1):84–90. [DOI] [PubMed] [Google Scholar]
- 32.Huret JL. Bone: aneurysmal bone cysts. Atlas Genet Cytogenet Oncol Haematol. 2012;16(5):368–371. [Google Scholar]
- 33.Lange T, Stehling C, Frohlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(6):1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papagelopoulos PJ, Currier BL, Shaughnessy WJ, et al. Aneurysmal bone cyst of the spine. Spine. 1998;23(5):621–628. [DOI] [PubMed] [Google Scholar]