Abstract
Lissencephaly is a severe brain malformation in which failure of neuronal migration results in agyria or pachygyria and in which the brain surface appears unusually smooth. It is often associated with microcephaly, profound intellectual disability, epilepsy, and impaired motor abilities. Twenty-two genes are associated with lissencephaly, accounting for approximately 80% of disease. Here we report on 12 individuals with a unique form of lissencephaly; these individuals come from eight unrelated families and have bi-allelic mutations in APC2, encoding adenomatous polyposis coli protein 2. Brain imaging studies demonstrate extensive posterior predominant lissencephaly, similar to PAFAH1B1-associated lissencephaly, as well as co-occurrence of subcortical heterotopia posterior to the caudate nuclei, “ribbon-like” heterotopia in the posterior frontal region, and dysplastic in-folding of the mesial occipital cortex. The established role of APC2 in integrating the actin and microtubule cytoskeletons to mediate cellular morphological changes suggests shared function with other lissencephaly-encoded cytoskeletal proteins such as α-N-catenin (CTNNA2) and platelet-activating factor acetylhydrolase 1b regulatory subunit 1 (PAFAH1B1, also known as LIS1). Our findings identify APC2 as a radiographically distinguishable recessive form of lissencephaly.
Keywords: APC2, lissencephaly, agyria, pachygyria, band heterotopia, epilepsy, intellectual disability, epilepsy, neuronal migration
Main Text
The development of the cerebral cortex is a complex dynamic process that occurs primarily between gestational weeks 6 and 20. The predominant steps include neural stem cell proliferation and differentiation, migration from the ventricular site of origin outward to the developing cortical plate, and cortical organization associated with synaptogenesis and neural network formation. Disruption of this process can lead to many different malformations of cortical development (MCD), the diversity of which is increasingly highlighted through advances in both brain imaging and molecular genetics.1 MCDs collectively represent a major cause of neurodevelopmental disorders; they are often associated with severe epilepsy and contribute to morbidity and mortality in the first decade of life.2 MCDs associated with defects in neuronal migration include the lissencephaly spectrum of disorders (LIS, agyria-pachygyria) and less severe MCDs, including subcortical band heterotopia and tubulinopathy-associated dysgyrias.
Although some types of MCDs can result from environmental factors, those in the LIS spectrum are almost always due to recessive, dominant, or X-linked mutations, encoding proteins that regulate the neuronal cytoskeleton (both actin and microtubules)—a critical function as neurons migrate. To date, genes associated with these disorders include ACTB (MIM: 102630), ACTG1 (MIM: 102560), ARX (MIM: 300382), CDK5 (MIM: 123831), CRADD (MIM: 603454), CTNNA2 (MIM: 114025), DCX (MIM: 300121), DYNC1H1 (MIM: 600112), KIF2A (MIM: 602591), KIF5C (MIM: 604593), PAFAH1B1 (also known as LIS1; MIM: 601545), MACF1 (MIM: 608271), MAST1 (MIM: 612256), NDE1 (MIM: 609449), RELN (MIM: 600514), TUBA1A (MIM: 602529), TUBB (MIM: 191130), TUBB2A (MIM: 615101), TUBB2B (MIM: 612850), TUBB3 (MIM: 602661), TUBG1 (MIM: 191135), and VLDLR (MIM: 192977), and they account for more than 80% of individuals with LIS and LIS variants.4, 5 Differences in the gyral pattern and associated brain malformations, or other non-brain dysmorphisms, make it possible to distinguish some genetic forms of LIS. Some genes associate with a posterior-predominant (i.e., P > A) LIS (PAFAH1B1, TUBA1A and others), and others with anterior-predominant (A > P) LIS (DCX, ACTB, RELN and others). The LIS gradient and associated brain and other malformations allow for distinction of at least 21 different subtypes of LIS and allow for prediction of likely mutant genes for newly identified individuals.5
In a collaborative effort to identify additional mechanisms underlying LIS, we recruited eight families for whom previous phenotypic and molecular analysis suggested a novel cause of the disorder. This cohort included families that could not be accurately classified into existing phenotypic categories or for whom testing for mutations in existing genes was negative. This study was performed within an ethical framework set by the University of California, San Diego IRB, and informed consent was obtained on each individual involved in this study. We recruited family 1 from Egypt; this family had documented parental 1st degree consanguinity and two affected male siblings who showed a nearly identical clinical pattern of severe developmental delay and myoclonic seizures starting at 5 months of age, along with a radiographic pattern of P > A LIS (Figures 1A and 1B). Genomic DNA from both affected individuals underwent whole-exome sequencing with the SureSelect Human All Exome 50 Mb kit (Agilent Technologies), and 125 bp paired-end read sequences were generated on a HiSeq2500 (Illumina), then analyzed according to GATK best practices (see Supplemental Methods). We identified a homozygous truncating p.Gln361∗ mutation in APC2 (MIM: 612034) (GenBank: NM_005883.2, UCSC Genome Browser: uc002lsr.1) encoding adenomatous polyposis coli protein 2, which is expressed throughout the central nervous system.6 Other homozygous variants in this family were relatively common, were less likely to damage protein function, or were already linked to other diseases; for example, KCNMA1 (MIM 600150) loss causes autosomal-dominant paroxysmal nonkinesigenic dyskinesia (MIM: 609446), and FBN1 (MIM: 134797) loss causes autosomal-dominant Marfan syndrome (MIM: 154700), which affected individuals do not have (Table S1).
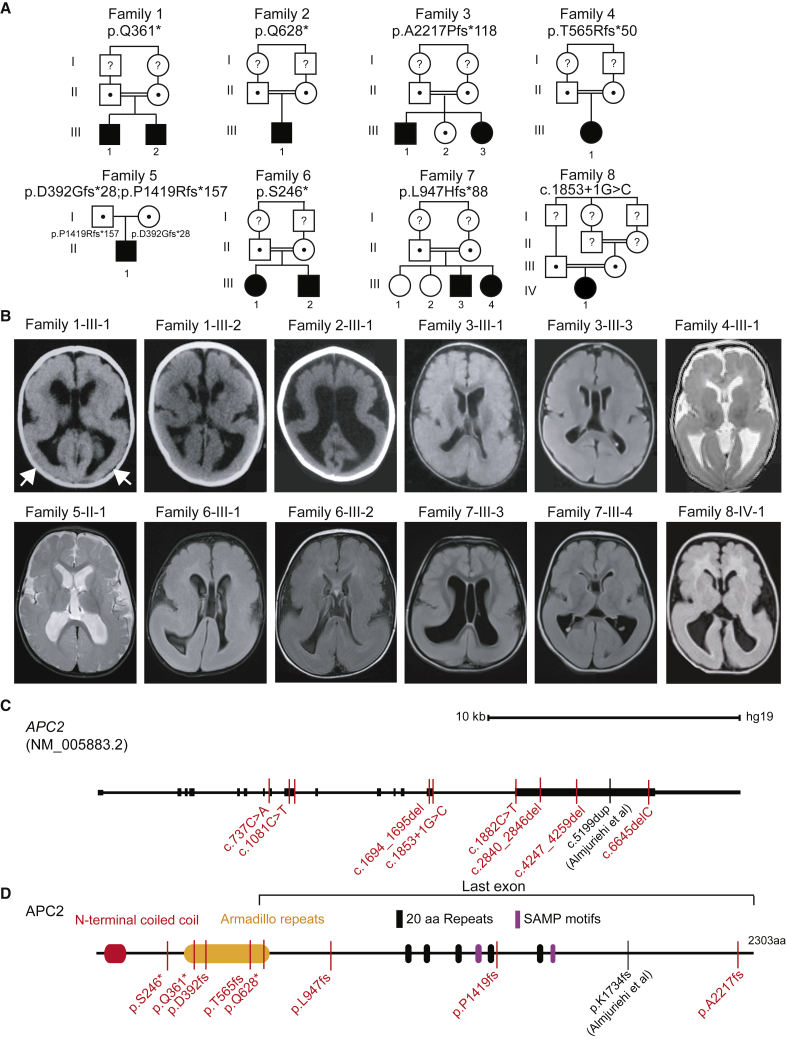
Figure 1.
APC2 Bi-allelic Loss of Function Mutations in Posterior-Predominant (P > A) Lissencephaly
(A) Twelve affected individuals from eight families showed unique bi-allelic mutations in APC2. All families except family 5 had documented parental consanguinity (double bars). The allele is listed below the family number. Dots and question marks indicate heterozygous carriers and samples not tested, respectively.
(B) Axial brain imaging in each family showed evidence of posterior-predominant lissencephaly. In families 1 and 2, only brain CT was available, but for other families, brain MRI is shown. Scans showed more severe agyria in the posterior than in the anterior cortex (arrows in first scan highlight posterior agyria). Scans are T1 or FLAIR sequences, except for those of families 4 and 5, which are T2 sequences.
(C) Gene organization of APC2. The scale bar represents 10 kb. APC2 contains 15 exons, the first of which is non-coding. Mutations were scattered throughout the coding region of the protein; five mutations were present in large exon 15. Exon 15 also contains the homozygous c.5199dup Almuriekhi et al. mutation.16
(D) APC2 is a 2,303 amino acid multidomain scaffolding protein containing an N-terminal coiled-coil, Armadillo repeats, 20 amino acid (aa) repeats (FXVEXTPXCFSRXSSLSSLS), and SAMP (Ser-Ala-Met-Pro) motifs. Affected individuals’ mutations, represented with simplified nomenclature, were located throughout the open reading frame. The region of the protein encoded by the last exon is highlighted.
Two other families from our lissencephaly cohort of 75 families with predominantly recessive MCDs also showed homozygous truncating mutations in APC2. Families 2 and 3 both had documented parental 1st degree consanguinity. Brain MRIs in both families showed a P > A LIS pattern that closely matched images of family 1. Family 2 had a single affected child and demonstrated a homozygous p.Gln628∗ mutation, whereas family 3 had two affected and one healthy child and demonstrated a homozygous p.Ala2217Profs∗118 mutation in the affected individuals. These data suggest that homozygous loss of function (LoF) mutations in APC2 lead to fully penetrant P > A LIS. Through Matchmaker Exchange and correspondence with colleagues, we identified five additional families in which there were truncating APC2 mutations that were independently identified as likely to be most relevant to clinical presentation (Table S1) and in which brain MRIs of affected individuals showed P > A LIS. The protein alterations included homozygous p.Thr565Argfs∗50, p.Ser246∗, p.Leu947Hisfs∗88 and c.1853+1G>C (splice donor), and compound heterozygous p.Asp392Glyfs∗28;p.Pro1419Argfs∗157. None of the eight families had damaging mutations in any known LIS genes. Thus, we identified a total of eight families that together included 12 individuals with bi-allelic APC2 LoF mutations and P > A LIS, suggesting bi-allelic loss of APC2 function as a rare cause of P > A LIS.
All affected children were born full term without any complications during pregnancy and delivery (Table 1). Although as a group length and weight at birth were normal, most affected individuals showed a trend toward smaller head circumference; only one individual met criteria for microcephaly, defined as a head size < 3 standard deviation (SD) below the mean, at the most recent measurement (Table 1). Most individuals presented at 3 months to 3 years of age with severe developmental delay, including absent or delayed milestones, and had seizures starting at 3 months to 5 years of age. Seizures were typically myoclonic or generalized tonic clonic and occurred daily to monthly. Electroencephalograms for most individuals showed generalized epileptiform activity. Neurological findings included hypotonia of the trunk and hypertonia of the extremities, along with alterations in deep tendon reflexes, features that are typical in severe LIS. None of the individuals were able to walk or had any language skills. Standard metabolic testing, visual evoked potentials, evaluation for dysmorphology, and review of organ systems were unremarkable in all tested individuals. Thus, clinical features do not distinguish APC2-LIS from the reported spectrum of typical severe LIS.
Table 1.
Clinical Features of Individuals with APC2 Mutations
| Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | Family 7 | Family 8 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | Egypt | Egypt | Egypt | Iran | USA | Turkey | Syria | Egypt | ||||
| Variant | ||||||||||||
| Zygosity | homozygous | homozygous | homozygous | homozygous | compound heterozygous | homozygous | homozygous | homozygous | ||||
| Genomic (hg19) | chr19: g.1457116C>T | chr19: g.1465182C>T | chr19: g.1469945delC | chr19: g.1462017_1462018delCA | chr19: g.1457202del; g.1467547del | chr19: g.1456324C>A | chr19 :g.1466140_1466146del | chr19: g.1462177G>C | ||||
| cDNA | c.1081C>T | c.1882C>T | c.6645delC | c.1694_1695delCA | c.1167_1180del; c.4247_4259del | c.737C>A | c.2840_2846del | c.1853+1G>C | ||||
| Protein | p.Gln361∗ | p.Gln628∗ | p.A2217fs∗118 | p.T565Rfs∗50 | p.D392Gfs∗; p.P1419Rfs*157 | p.Ser246∗ | p.L947Hfs∗88 | Splice donor | ||||
| Proband | 1-III-1 | 1-III-2 | 2-III-1 | 3-III-1 | 3-III-3 | 4-III-1 | 5-II-1 | 6-III-1 | 6-III-2 | 7-III-3 | 7-III-4 | 8-IV-1 |
| Gender | M | M | M | M | F | F | M | F | M | M | F | F |
| Weight at birth (kg) | 3.2 (−0.5 SD) | 3 (−1 SD) | 3.5 (mean) | 3 (−1 SD) | 3.2 (−0.3 SD) | 3.9 (mean) | 10–25 centiles | 2.9 (−1.0 SD) | 3 (−0.9 SD) | ∼2 | ∼2 | NA (normal) |
| Length at birth (cm) | 50 (mean) | 49 (−0.2 SD) | 49 (−0.2 SD) | 50 mean | 48 (−0.2 SD) | 50 (mean) | 10–25 centiles | 48 (−0.7 SD) | 49 (−0.5 SD) | NA | NA | NA (normal) |
| HC at birth (cm) | 35 (−0.5 SD) | 34.5 (−0.8 SD) | 34.5 (−0.8 SD) | 35 (−0.5 SD) | 34 (−0.8 SD) | 37 (mean) | 90–95 centiles | 34 (−0.4 SD) | 34 (−0.6 SD) | NA | NA | NA (normal) |
| Age at last examination | 2 years | 9 months | 2 years | 15 years | 5 years | 3 years | 7.5 years | 4 years | 2 years | 4 years, 7 months | 6 years | 7 months |
| HC at last examination (cm) | 48.5 (−0.1 SD) | 43.5 (−1.4SD) | 49 (mean) | 51 (−2.6SD) | 48.5 (−1.3SD) | 45 (−3.8 SD) | NA | 47 (−2.1SD) | 44.5 (−3.1SD) | 47 (−2.9 SD) | 47 (−SD) | NA |
| Diagnosis age | 3 years | 3 months | 2 years | 2 years | 6 months | 3 months | 19 months | 18 months | 8 months | 6 months | 4 months | 7 months |
| Intellectual Disability | severe | severe | severe | severe | severe | severe | severe | severe | severe | severe | severe | severe |
| Psychomotor Development | ||||||||||||
| Gross motor | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed, no head control |
| Fine motor | absent | absent | absent | absent | absent | absent | delayed | delayed | delayed | delayed | delayed | delayed |
| Language | delayed | delayed | delayed | absent | absent | delayed | delayed | delayed | delayed | absent | absent | NA |
| Social | delayed | delayed | delayed | delayed | delayed | delayed | unknown | delayed | delayed | delayed | delayed | delayed |
| Seizures | Y | Y | Y | Y | Y | N | N | Y | Y | Y | N | N |
| Age of Onset | 5 months | 3 months | 4 months | 6 years | 4.5 years | − | − | 12 months | 18 months | 2 years | − | − |
| Type | generalized and myoclonic | generalized and myoclonic | myoclonic seizure and infantile spasm | generalized and myoclonic | generalized and myoclonic | − | − | generalized and myoclonic | generalized and myoclonic | generalized and myoclonic | − | − |
| Frequency | monthly | monthly | daily | with fever | daily | − | − | daily | daily | weekly | − | − |
| Controlled/Refractory | fairly controlled | fairly controlled | refractory | controlled | refractory | − | − | refractory | refractory | controlled | − | − |
| EEG | generalized epileptogenic activity involving midline structure | generalized epileptogenic activity | hypsarrhythmia | bilateral tempro-pariental epileptogenic activity | generalized epileptoggenic activity | normal | normal | generalized epileptogenic activity | generalized epileptogenic activity | NA | NA | NA |
| Neurological Findings | ||||||||||||
| Hypertonia | Y | Y | N | N | N | N | N | N | N | Y, peripheral | Y, peripheral | N |
| Hypotonia | N | N | Y | Y | Y | Y, truncal | Y | Y | Y | Y, central | Y, central | Y |
| Spastic tetraplegia | Y | Y | tetraplegia but not spastic | N | N | spastic dystonia | NA | N | N | Y | N | N |
| Investigations | ||||||||||||
| Metabolic | normal | normal | normal | normal | normal | normal | NA | normal | normal | normal | normal | NA |
| VEP and ERG | normal | normal | normal | normal | normal | normal | NA | NA | NA | NA | NA | NA |
| Neuroimaging | CT | CT | CT | MRI | MRI | MRI | MRI | MRI | MRI | MRI | MRI | MRI |
| P > A Lissencephaly | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Cerebral mantle thickening | >1 cm | >1 cm | unknown | >1 cm | >1 cm | 8–10 mm | NA | Y | Y | Y | Y | >1 cm |
| Ribbon heterotopia | NA | NA | NA | N | N | N | Y | Y | Y | Y | Y | N |
| Corpus callosum hypogenesis | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Cerebellar hypoplasia | N | N | N | N | N | very mild | N | very mild | N | N | N | N |
| Brainstem hypoplasia | N | N | N | N | N | N | N | very mild | Y | small pons | small pons | N |
| Ventriculomegly | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | mild |
| White matter paucity | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Genomic position of allele is presented in hg19 reference. Abbreviations are as follows: cm, centimeter; F, female; M, male; HC, head circumference; L, left; MRI, magnetic resonance imaging; NA, not available; −, negative; R, right; SD, standard deviation; VEP, visual evoked potential; and ERG, electroretinogram.
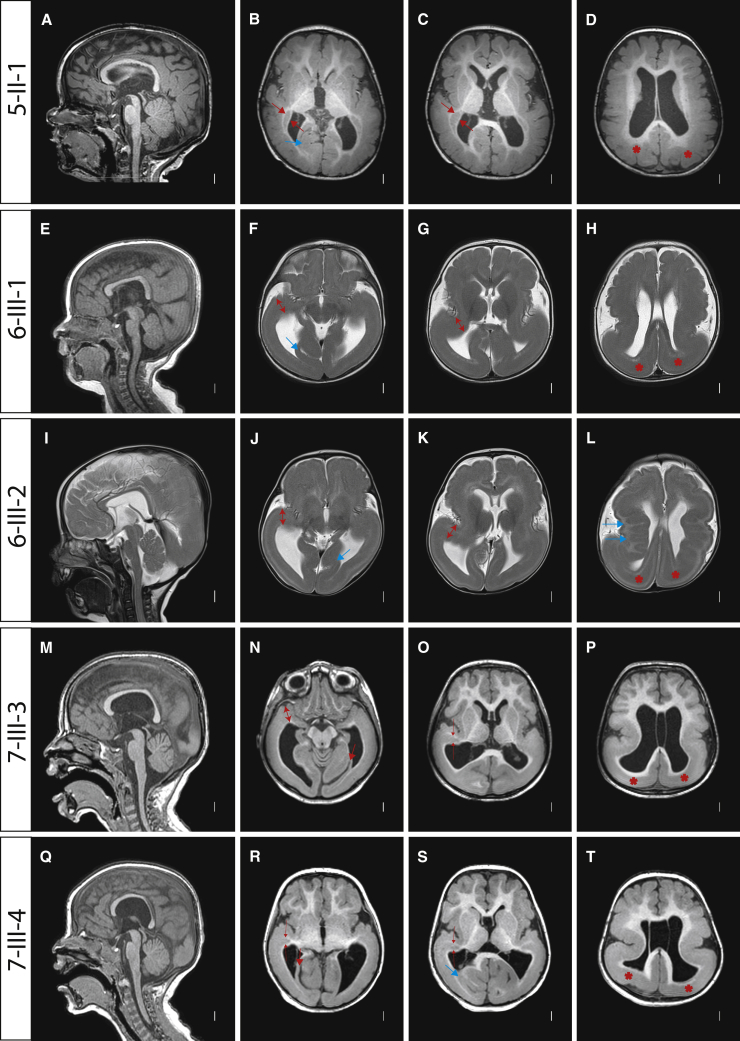
In five of the individuals, high-resolution brain MRIs were available for review (Figure 2), and these demonstrated features not common in other causes of LIS. All individuals demonstrated a P > A gradient, which has been reported with PAFAH1B1, TUBG1, ARX, DYNC1H1 and can also be seen in association with TUBA1A, TUBB2B, KIF5C, and KIF2A.5 In addition, ventriculomegaly with stretched and thinned corpus callosum and an unusual posterior subcortical heterotopia just posterior to the caudate nuclei were noted in several individuals. In some individuals, the subcortical heterotopia appeared to merge with the deep cellular layer of the posterior agyria, and almost no white matter was visible. One child (6-III-2) had an undulating, ribbon-like deep cellular layer (Figure 2L) that began in the mid-frontal lobe and continued posteriorly to the parietal lobe, in a pattern that was very different from the subcortical band heterotopia (MIM: 300067) seen with DCX mutations. The ribbon-shaped subcortical heterotopia of subject 6-III-2 was reminiscent of the ribbon-like heterotopia observed with EML1 mutations (MIM: 600348).7 However, EML1 mutations are also associated with hydrocephalus, agenesis of the corpus callosum, and diffuse polymicrogyria. Additionally, with CENPJ mutation (MIM: 608393), a more discrete thin festooned heterotopia in the areas lateral and adjacent to the striatum has been seen (W.B.D. personal observation), and thin subcortical heterotopia band in the upper frontal area and parallel to the lateral ventricles is reported with GPSM2 mutations causing Chudley-McCullough syndrome (MIM: 604213).8 However, the pattern of subcortical heterotopia seen in these conditions is different from that of APC2. In addition, all subjects showed hippocampal defects (Figure S1) and striking dysplastic in-folding of the mesial occipital cortex, neither of which are seen in other forms of LIS (Figure 2; examples can be seen in Figures 2B, 2F, 2J, 2N, and 2R). In fact, recognizable brain MRI findings (W.B.D., unpublished data) ultimately led to the clinical diagnosis of the affected individual from family 8 as most likely harboring APC2. Thus, we believe that the images can distinguish APC2-LIS from other forms of disease.
Figure 2.
Posterior-Predominant (P > A) LIS with Subcortical Ribbon Heterotopia Associated with Bi-allelic APC2 Mutations
Individual identifier along left. Midline sagittal MRIs showed P > A LIS with a stretched and thinned corpus callosum and relatively well-preserved anterior folding, brainstem, and cerebellar architecture. Axial images all showed P > A LIS with mild (B–D, N–P, and R–T) or moderate (F–H and J–L) frontal pachygyria and posterior agyria (asterisks shown in fourth column only). 5-II-1: short, comma-shaped subcortical heterotopias began just posterior to and at the same level as the tail of the caudate nuclei (between the red arrows in [B and C]). 7-III-3 and 7-III-4: same subcortical heterotopia began in the same place, but then merged with the deep cellular layer of the posterior agyria (thin red arrows in O, R, and S). 6-III-1 and 6-III-2 (and in [R]): this same region appeared to have dysplastic cortex extending from the pial surface to the ventricular surface, with no white matter apparent (two-headed arrows in [F], [G], [J], [K], and [N]). The ribbon heterotopia began at this level (two-headed arrow in [K]). All subjects showed striking dysplastic in-folding of one or several gyri in the mesial occipital region (thick blue or red arrows in all five images in second column). The selected images include T1-weighted (A–D and M–T) and T2-weighted (E–L) images in the midline sagittal (first column) and multiple axial planes progressed from low to high slices (second to fourth columns).
The adenomatous polyposis coli gene family (not to be confused with the multisubunit anaphase promoting complex) consists of two paralogs conserved to Drosophila, APC (MIM: 611730) and APC2. APC was first identified as a human colon cancer tumor suppressor, associated with both sporadic and inherited forms of the disease,9 and APC functions as a negative regulator of Wnt signaling and in the organization and regulation of the actin and microtubule cytoskeletons.10 APC2 (also called APCL) is highly similar to APC in its N-terminal armadillo-repeat containing half, but it shares little sequence similarity to its C-terminal half. APC2 is not mutated in colon cancer, binds less efficiently to β-catenin than APC, and has not been implicated in Wnt signaling.11, 12 APC2 localizes to actin and microtubule fibers, and Apc2−/− mice show disrupted neuronal migration, leading to defects in lamination of the cerebral cortex and cerebellum13 and thus supporting APC2 as a LIS candidate gene.
Encoded on human chromosome 19, APC2 consists of 15 coding exons and a 10.1 kb coding mRNA. Three alternative splice isoforms are described, but the major isoform encodes a 2,303 amino acid protein. We identified truncating mutations in four of these exons, and we found that most occurred in the largest and last exon, exon 15 (Figure 1C). The four truncating mutations identified in the last exon were predicted to lead to a stable mRNA and potentially a C-terminally truncated protein, whereas mutations in earlier exons were predicted to lead to nonsense mediated decay and LoF. We considered the possibility that late truncating mutations might have a milder phenotype than early truncating mutations, but we found no evidence of milder clinical or radiographic phenotypes. The locations of the truncating mutations occurred throughout the open reading frame (Figure 1D), and the lack of correlation of the location with the severity of the imaging phenotype suggested that most or all of these mutations are LoF. The variants were unique in our dataset of >5,000 exomes from individuals with neurodevelopmental phenotypes and were not represented in the Greater Middle Eastern Variome, 1000 Genomes, or gnomAD databases (Table S1). All variants were confirmed by Sanger sequencing and segregated according to a recessive mode of inheritance.
APC2 has pLI value of 1.0 in gnomAD, suggesting haploinsufficiency intolerance. However, heterozygous carriers in this study did not have any noticeable phenotype. Constraint metrics such as pLI and the more recently introduced o/e ratio represent a spectrum of tolerance to inactivation.14 Although pLI is generally accepted as an indicator of LoF intolerance, not all genes with a high pLI score cause disease, even if they have heterozygous LoF variants. Thus, a pLI of 1.0 of APC2 does not necessarily mean that heterozygous LoF variants of APC2 cause haploinsufficiency or disease. In fact, 27 heterozygous APC2 variants that have been predicted to be LoF with high confidence were found in gnomAD in apparently healthy individuals. This means that heterozygous LoF variants of APC2 are probably not sufficient to produce disease, might produce disease in specific genetic backgrounds, or might be subject to purifying selection on the population rather than the individual level.15 Therefore, heterozygous carriers in our study might have an unnoticeable phenotype, although they were not examined by brain MRI.
A recent publication identified a late truncating homozygous single-base duplication (p.Lys1734Glnfs∗419; Figures 1C and 1D) in exon 15 of APC2 in two affected children from a consanguineous marriage. These children displayed Sotos-like features but had no noted brain malformations (MIM: 617169).16 Sotos syndrome is a form of cerebral gigantism and is associated with intellectual disability and macrocephaly (MIM: 117550). The children showed developmental delay and macrocephaly, and brain MRIs showed only dilated brain ventricles. We reviewed the brain MRIs in the published paper and found no evidence of LIS. The reported variant was predicted to lead to replacement of the C-terminal 570 amino acids with 418 aberrant residues. Despite the fact that all of our affected individuals had bi-allelic truncating mutations throughout the protein, none of our subjects showed macrocephaly or Sotos-like features. This leaves the open question as to why this reported homozygous frameshift variant did not produce LIS. Possibilities include (1) that the variant did not fully inactivate the protein, (2) that it produced a novel function, and (3) that it is an allele-specific association.17 Determining the full phenotypic spectrum associated with APC2 mutations as additional individuals and alleles are identified will require further work.
The role of APC2 in LIS remains to be established, but the phenotypes we report together with APC2’s published localization and binding partners support functional interaction with other LIS-related proteins. In migratory neurons, APC2 partially co-localizes with microtubules and F-actin at the leading edge of the growth cone.13 In Apc2−/− neurons, BDNF stimulation fails to increase the amount of F-actin at the leading edge or effectively stabilize microtubules.
We recently reported on homozygous CTNNA2 mutations in LIS, and like APC2, the CTNNA2-encoded protein (α-N-catenin) can interact with both β-catenin and actin. In CTNNA2-related LIS, defects in Wnt signaling were excluded; instead, α-N-catenin competed with the Arp2/3 complex to suppress actin branching,18 leading to more stable leading neurites. Another recent report linked APC to cytoplasmic dynein through the cofactor AMER (APC-membrane recruitment) family of membrane-bound proteins.19 Furthermore, APC has been reported to play an important role in regulation of radial glial polarity and interneuron migration by modulating microtubule severing and to be essential for cortex development.20, 21 APC2 might similarly serve as a microtubule regulator or form a complex with α-N-catenin or dynein to mediate neurite stability or the minus-end-directed dynein forces required during migration, although further experimental studies should follow to support these speculations. Interestingly, a genetic interaction between Pafah1b1 and Apc in murine neuronal migration was reported, but investigations of Apc2 were not performed.22 Finally, in postmitotic neurons, APC2 controls dendritic development by promoting microtubule dynamics through two separate microtubule binding domains.23 It is possible that these domains function during neuronal migration to mediate leading-process organization.
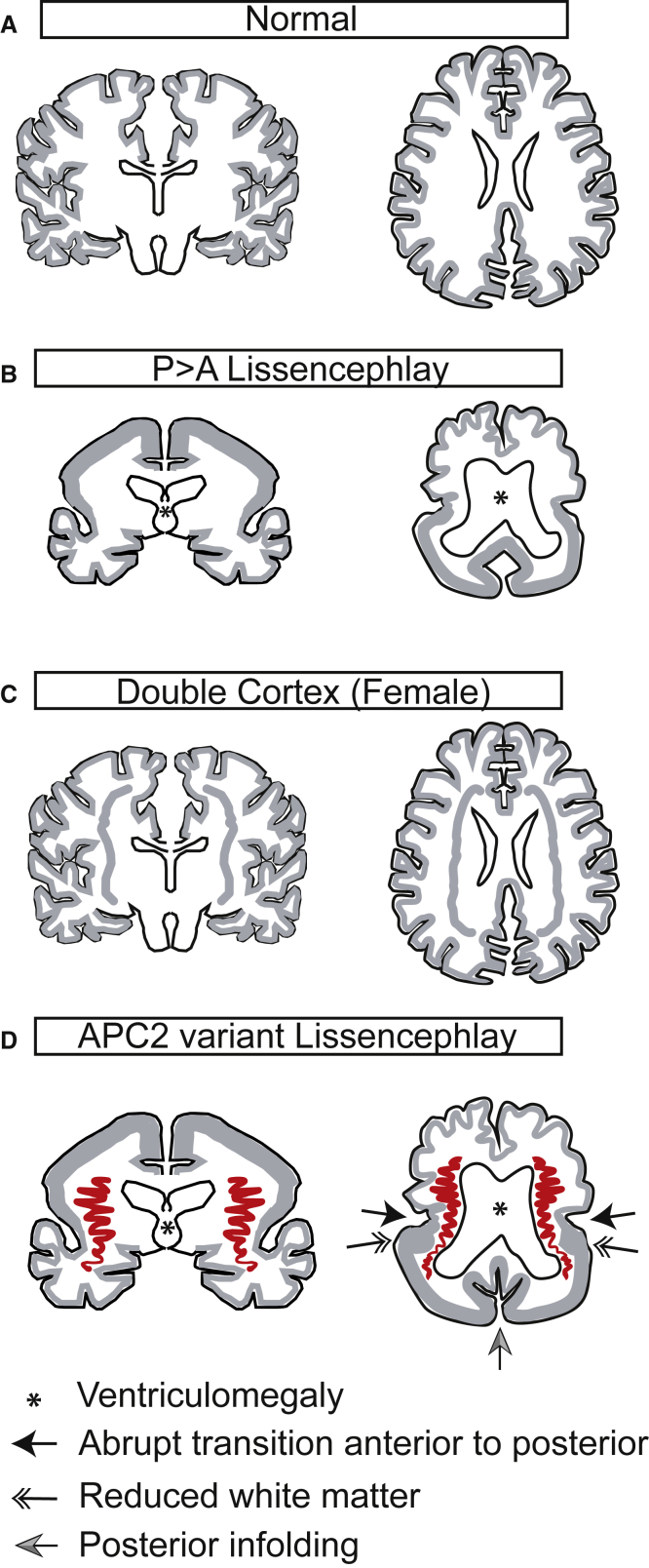
In summary, we implicate APC2 in a recessive form of P > A LIS, clinically characterized by severe intellectual disability, epilepsy, and neuromotor involvement and radiographically characterized by a stretched and thinned corpus callosum, subcortical thin and sometimes ribbon-shaped heterotopia in posterior perisylvian areas, and dysplastic in-folding of gyri in the mesial occipital cortex (Figure 3). There are most likely a range of developmental brain phenotypes resulting from loss of APC2, although our subjects are likely to be at the most severe end of the spectrum given the nature of the alleles. Elucidating the full range of phenotypes, genotype-phenotype correlations, and mechanisms of pathogenicity will require future studies.
Figure 3.
Schematic Depiction of Major Migrational Defects in Lissencephaly Subtypes
(A) The normal type shows evenly spaced cortical gyri and sulci and a thin mantle of gray matter.
(B) The P > A lissencephaly subtype shows thickened cortical gray matter mantle in the neocortex (left); this gray matter is more severe in the posterior cortex (right). Ventriculomegaly is also depicted (∗).
(C) The double-cortex subtype shows a normally gyrated outer cortex with a normal cortical mantle, but additionally it shows a band heterotopia, which is evenly distributed anteriorly and posteriorly in the subcortical white matter.
(D) APC2 lissencephaly shows P > A gradient with a thickened cortical gray matter mantle in the posterior and relatively preserved gyration in the anterior; the transition is abrupt (arrow). In the temporal region, the dysplastic cortex extends from the pia to the ventricle, resulting in reduced white matter (double arrow). Ribbon-like heterotopia is most noticeable in the perisylvian region and appears to connect with the tail of the caudate nuclei (red). In-folding of cortex in the mesial occipital region is often apparent (gray arrow).
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We thank the children and their families for their contributions to this study. This work was supported by National Institutes of Health grants U01 MH108898, R01 NS048453, R01 NS098004, the Simons Foundation Autism Research Initiative (SFARI), the Howard Hughes Medical Institute (J.G.G.), Qatar National Research Foundation 6-1463 (J.G.G.), and the March of Dimes 6-FY14-422 (M.C.M.). G.M.S.M. is supported by the ZonMW TOP grant 91217045. N.D.D., U.A., W.B.D., G.M.M., G.M.S.M., and M.S.Z. are members of the European Network on Brain Malformations (Neuro-MIG, European Cooperation in Science and Technology [COST] Action CA16118). T.K. was supported by COST Action CA16118 (STSM grant #41344). G.M.M. is supported by National Institutes of Health grant K08NS092898 and Jordan’s Guardian Angels. Data on one family was collected as part of the SYNaPS Study Group collaboration funded by The Wellcome Trust and strategic award (Synaptopathies) funding (WT093205 MA and WT104033AIA). This research was conducted as part of the University College London Queen Square Genomics group, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. We thank the Rady Children’s Institute for Genomic Medicine, the Broad Institute (U54HG003067 to E. Lander and UM1HG008900 to D. MacArthur), the Yale Center for Mendelian Disorders (U54HG006504 to R. Lifton and Murat Gunel) for sequencing support, and the Matchmaker Exchange. We acknowledge M. Gerstein, S. Mane, A.B. Ekici, S. Uebe, E.S. Cauley, and the University of California, San Diego Institute for Genomic Medicine Genetics Center for sequencing support and analysis, the Yale Biomedical High-Performance Computing Center for data analysis and storage, the Yale Program on Neurogenetics, and the Yale Center for Human Genetics.
Published: October 3, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.08.013.
Web Resources
1000 Genomes http://www.1000genomes.org/
GeneReviews, Bahi-Buisson, N., and Cavallin, M. (1993). Tubulinopathies Overview. https://www.ncbi.nlm.nih.gov/books/NBK350554/
Greater Middle East Variome Project, http://igm.ucsd.edu/gme/
Matchmaker Exchange https://www.matchmakerexchange.org/
OMIM http://www.omim.org/
UniProt http://www.uniprot.org/uniprot/
Supplemental Data
References
- 1.Guerrini R., Dobyns W.B., Barkovich A.J. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–162. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Parrini E., Conti V., Dobyns W.B., Guerrini R. Genetic basis of brain malformations. Mol. Syndromol. 2016;7:220–233. doi: 10.1159/000448639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Donato N., Kuechler A., Vergano S., Heinritz W., Bodurtha J., Merchant S.R., Breningstall G., Ladda R., Sell S., Altmüller J. Update on the ACTG1-associated Baraitser-Winter cerebrofrontofacial syndrome. Am. J. Med. Genet. A. 2016;170:2644–2651. doi: 10.1002/ajmg.a.37771. [DOI] [PubMed] [Google Scholar]
- 5.Di Donato N., Timms A.E., Aldinger K.A., Mirzaa G.M., Bennett J.T., Collins S., Olds C., Mei D., Chiari S., Carvill G. Analysis of 17 genes detects mutations in 81% of 811 patients with lissencephaly. Genet. Med. 2018;20:1354–1364. doi: 10.1038/gim.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Es J.H., Kirkpatrick C., van de Wetering M., Molenaar M., Miles A., Kuipers J., Destrée O., Peifer M., Clevers H. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr. Biol. 1999;9:105–108. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- 7.Kielar M., Tuy F.P., Bizzotto S., Lebrand C., de Juan Romero C., Poirier K., Oegema R., Mancini G.M., Bahi-Buisson N., Olaso R. Mutations in Eml1 lead to ectopic progenitors and neuronal heterotopia in mouse and human. Nat. Neurosci. 2014;17:923–933. doi: 10.1038/nn.3729. [DOI] [PubMed] [Google Scholar]
- 8.Kau T., Veraguth D., Schiegl H., Scheer I., Boltshauser E. Chudley-McCullough syndrome: case report and review of the neuroimaging spectrum. Neuropediatrics. 2012;43:44–47. doi: 10.1055/s-0032-1307451. [DOI] [PubMed] [Google Scholar]
- 9.Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 10.Aoki K., Taketo M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 11.Schneikert J., Vijaya Chandra S.H., Ruppert J.G., Ray S., Wenzel E.M., Behrens J. Functional comparison of human adenomatous polyposis coli (APC) and APC-like in targeting beta-catenin for degradation. PLoS ONE. 2013;8:e68072. doi: 10.1371/journal.pone.0068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M.N., Kunttas-Tatli E., Zimmerman S., Zhouzheng F., McCartney B.M. Cortical localization of APC2 plays a role in actin organization but not in Wnt signaling in Drosophila. J. Cell Sci. 2011;124:1589–1600. doi: 10.1242/jcs.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shintani T., Takeuchi Y., Fujikawa A., Noda M. Directional neuronal migration is impaired in mice lacking adenomatous polyposis coli 2. J. Neurosci. 2012;32:6468–6484. doi: 10.1523/JNEUROSCI.0590-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. 2019 doi: 10.1101/531210. [DOI] [Google Scholar]
- 15.Cassa C.A., Weghorn D., Balick D.J., Jordan D.M., Nusinow D., Samocha K.E., O’Donnell-Luria A., MacArthur D.G., Daly M.J., Beier D.R., Sunyaev S.R. Estimating the selective effects of heterozygous protein-truncating variants from human exome data. Nat. Genet. 2017;49:806–810. doi: 10.1038/ng.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almuriekhi M., Shintani T., Fahiminiya S., Fujikawa A., Kuboyama K., Takeuchi Y., Nawaz Z., Nadaf J., Kamel H., Kitam A.K. Loss-of-function mutation in APC2 causes Sotos syndrome features. Cell Rep. 2015;10:1585–1598. doi: 10.1016/j.celrep.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 17.De Franco E., Watson R.A., Weninger W.J., Wong C.C., Flanagan S.E., Caswell R., Green A., Tudor C., Lelliott C.J., Geyer S.H. A specific CNOT1 mutation results in a novel syndrome of pancreatic agenesis and holoprosencephaly through impaired pancreatic and neurological development. Am. J. Hum. Genet. 2019;104:985–989. doi: 10.1016/j.ajhg.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaffer A.E., Breuss M.W., Caglayan A.O., Al-Sanaa N., Al-Abdulwahed H.Y., Kaymakçalan H., Yılmaz C., Zaki M.S., Rosti R.O., Copeland B. Biallelic loss of human CTNNA2, encoding αN-catenin, leads to ARP2/3 complex overactivity and disordered cortical neuronal migration. Nat. Genet. 2018;50:1093–1101. doi: 10.1038/s41588-018-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F.J., Shi L., Hines T., Hebbar S., Neufeld K.L., Smith D.S. Insulin signaling regulates a functional interaction between adenomatous polyposis coli and cytoplasmic dynein. Mol. Biol. Cell. 2017;28:587–599. doi: 10.1091/mbc.E16-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokota Y., Kim W.Y., Chen Y., Wang X., Stanco A., Komuro Y., Snider W., Anton E.S. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eom T.Y., Stanco A., Guo J., Wilkins G., Deslauriers D., Yan J., Monckton C., Blair J., Oon E., Perez A. Differential regulation of microtubule severing by APC underlies distinct patterns of projection neuron and interneuron migration. Dev. Cell. 2014;31:677–689. doi: 10.1016/j.devcel.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebbar S., Guillotte A.M., Mesngon M.T., Zhou Q., Wynshaw-Boris A., Smith D.S. Genetic enhancement of the Lis1+/- phenotype by a heterozygous mutation in the adenomatous polyposis coli gene. Dev. Neurosci. 2008;30:157–170. doi: 10.1159/000109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn O.I., Schätzle P., van de Willige D., Tas R.P., Lindhout F.W., Portegies S., Kapitein L.C., Hoogenraad C.C. APC2 controls dendrite development by promoting microtubule dynamics. Nat. Commun. 2018;9:2773. doi: 10.1038/s41467-018-05124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.