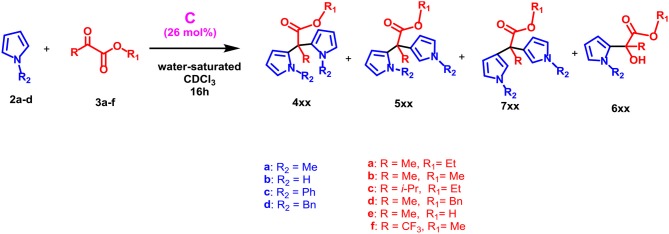
Table 2.
Scope of the reaction between different pyrroles 2a–d and α-ketoesters 3a–f.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entrya | Capsule | 2 | 3 | Yield (%)b | % (4xx)c | % (5xx)c | % (6xx)c | % (7xx)c |
| No | — | — | — | — | — | |||
| 1 | 2a | 3a | ||||||
| Yes | 98 | 60 (4aa) | 10 (5aa) | 28 (6aa) | — | |||
| No | — | — | — | — | — | |||
| 2d | 2a | 3b | ||||||
| Yes | 99 | 90 (4ab) | — | — | — | |||
| No | — | — | — | — | — | |||
| 3 | 2a | 3c | ||||||
| Yes | 55 | — | — | 55 (6ac) | — | |||
| No | — | — | — | — | — | |||
| 4 | 2a | 3d | ||||||
| Yes | 76 | 38 (4ad) | 38 (5ad) | — | — | |||
| No | — | — | — | — | — | |||
| 5e | 2a | 3e | ||||||
| Yes | 64 | — | — | — | — | |||
| No | 35 | — | — | 35 (6af) | — | |||
| 6 | 2a | 3f | ||||||
| Yes | 99 | — | — | 99 (6af) | — | |||
| No | — | — | — | — | — | |||
| 7 | 2b | 3a | ||||||
| Yes | 99 | 99 (4ba) | — | — | — | |||
| No | 38 | — | — | 38 (6bf) | — | |||
| 8 | 2b | 3f | ||||||
| Yes | 98 | — | — | 98 (6bf) | — | |||
| No | — | — | — | — | — | |||
| 9 | 2c | 3a | ||||||
| Yes | 50 | — | — | — | 50 (7ca) | |||
| No | — | — | — | — | — | |||
| 10 | 2d | 3a | ||||||
| Yes | 65 | — | — | 65 (6da) | — | |||
Reactions were performed on a 0.16 mmol scale using 2a–d (4 equiv.), 3a–e (1 equiv.), and capsule C (0.26 equiv.) in water saturated CDCl3 (1.1 mL) under stirring for 16 h at 30°C.
Overall yield of all the isolated products.
Yields of the isolated products by chromatography on column.
9% of adduct of pyrrole with two molecules of pyruvate is present; see Supporting Information.
Decarboxylated product is present, see Supporting Information.
