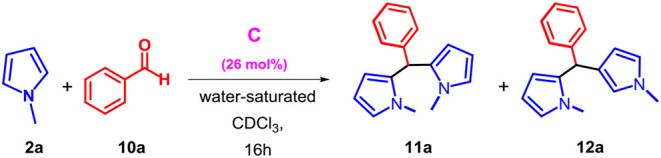
Table 4.
Optimization of reaction conditions for the reaction between 2a and 10a.
 | ||||||
|---|---|---|---|---|---|---|
| Entrya | Capsule | T (°C) | 2a/10a | Yield (%)b | 11a (%)c | 12a (%)c |
| 1 | No | 50°C | 1/1 | — | — | — |
| Yes | 38 | 34 | 4d | |||
| 2 | No | 50°C | 2/1 | — | — | — |
| Yes | 60 | 54 | 6 | |||
| 3 | No | 50°C | 4/1 | — | — | — |
| Yes | 97 | 87 | 10 | |||
| 4 | No | 25°C | 4/1 | — | — | — |
| Yes | 20 | 18 | 2d | |||
Reactions were performed on a 0.16 mmol scale using 2a (from 1 to 4 equiv.), 3a (1 equiv.), and capsule C (0.26 equiv.) in water saturated CDCl3 (1.1 mL) under stirring for 16 h.
Overall yield of all the isolated products.
Yields of the isolated products by chromatography on column.
The column gave an inseparable mixture with regioisomer and the yield was calculated by integration of the respective 1H-NMR signals of the regioisomers in the isolated fraction.
