Table 5.
Scope of the reaction with different pyrroles 2a–d and aldehydes 10a–j.
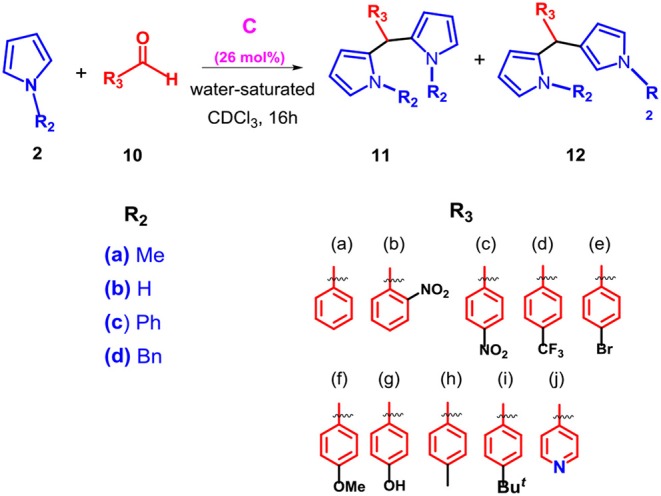 | ||||||
|---|---|---|---|---|---|---|
| Entrya | Capsule | 2 | 10 | Yield(%)b | % (11)c | % (12)c |
| 1 | No | 2b | 10a | — | — | — |
| Yes | 70 | 70 (11ba)e | — | |||
| 2 | No | 2c | 10a | — | — | — |
| Yes | — | — | — | |||
| 3 | No | 2d | 10a | — | — | — |
| Yes | — | — | — | |||
| 4 | No | 2a | 10b | — | — | — |
| Yes | 99 | 90 (11ab) | 9 (12ab) | |||
| 5 | No | 2a | 10c | — | — | — |
| Yes | 98 | 96 (11ac) | 2 (12ac)d | |||
| 6 | No | 2a | 10d | — | — | — |
| Yes | 98 | 88 (11ad) | 10 (12ad) | |||
| 7 | No | 2a | 10e | — | — | — |
| Yes | 95 | 93 (11ae) | 2 (11ae)d | |||
| 8f | No | 2a | 10f | — | — | — |
| Yes | 98 | 96 (11af) | 2 (12af)d | |||
| 9f | No | 2a | 10g | — | — | — |
| Yes | 98 | 96 (11ag) | 2 (12ag)d | |||
| 10 | No | 2a | 10h | — | — | — |
| Yes | 97 | 95 (11ah) | 2 (12ah)d | |||
| 11 | No | 2a | 10i | — | — | 6 (12ai) |
| Yes | 97 | 91 (11ai) | ||||
| 12 | No | 2a | 10j | — | — | — |
| Yes | 85 | 76 (11aj)d | 9 (12aj)d | |||
Reactions were performed on a 0.16 mmol scale using 2a–d (4 equiv.), 10a–j (1 equiv.), and capsule C (0.26 equiv.) in water saturated CDCl3 (1.1 mL) under stirring for 16 h at 50°C.
Overall yield of all the isolated products.
Yields of the isolated products by chromatography on column.
The column gave an inseparable mixture with the regioisomer and the yield was calculated by integration of the respective 1H-NMR signals of the regioisomers in the isolated fraction.
1H NMR spectrum on crude reaction mixture showed presence of other species obtained after chromathography purification as a complex and inseparable fraction not characterized.
These reactions were performed under stirring for 48 h at 50°C.
