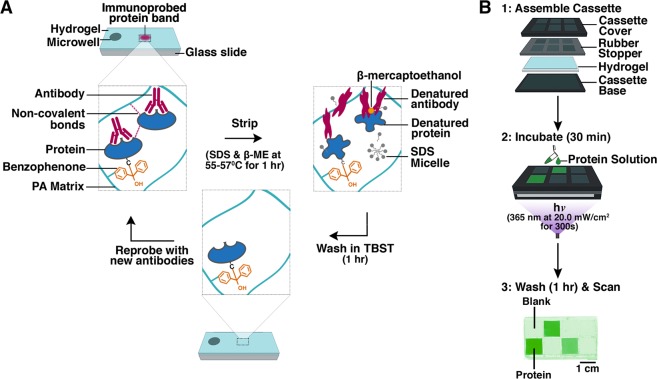
Figure 1.
Hypothesized mechanism of immunoassay signal loss in BMPA hydrogels and device fabrication to test hypothesis. (A) In BMPA hydrogels, protein targets of interest are covalently bonded to the hydrogel matrix by a benzophenone moiety. However, we hypothesize that some protein targets are immobilized in the gel matrix via non-covalent interactions (e.g., hydrogen bonding, hydrophobic bonding, or van der Waals forces). We hypothesize that the chemical stripping procedure used in multiplexing for hydrogel-based heterogeneous immunoassays denatures these non-covalent interactions, resulting in protein loss from the hydrogel. (B) To monitor and quantify protein loss from BMPA hydrogels during chemical stripping & reprobing, we used an ArrayIt Microarray Gasket to create physically isolated 1 cm2 arrays in the hydrogel. We loaded purified protein conjugated with fluorescent dye into pre-selected arrays, and filled remaining arrays with buffer. After a 30 min incubation step, gels are exposed to UV irradiation (365 nm, at 20.0 mW/cm2) for 300 s, and are subsequently washed and dried. Gels are scanned with a laser microarray scanner to measure fluorescence. We can now test our hypothesis by subjecting these hydrogels to multiple rounds of stripping and reprobing, wherein protein-conjugate fluorescence is used as a proxy for protein concentration, which allows us to monitor protein loss from the hydrogel across serial stripping rounds.