Abstract
Background
It is unclear whether high‐sensitivity troponin T (hs‐TnT) is associated with subclinical cardiac changes in chronic kidney disease (CKD). We evaluated the relationship between hs‐TnT and left ventricular structure and function in a CKD population, according to estimated glomerular filtration rate.
Methods and Results
We analyzed 2017 patients with CKD stages 1 to 5 (predialysis) in the KNOW‐CKD (Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease) cohort. The predictor was hs‐TnT level measured at baseline, and the outcomes were left ventricular hypertrophy (LVH) and systolic and diastolic dysfunction shown by echocardiography at baseline and after 4 years. Participants were categorized into quartiles according to hs‐TnT levels. The associations between quartiles of hs‐TnT and outcomes were assessed using multivariable logistic regression analysis with confounders including demographics, medical history, and laboratory findings. A receiver operating characteristic curve was used to assess the diagnostic power of hs‐TnT for the outcomes as a continuous variable. For subgroup analysis, patients were stratified based on an estimated glomerular filtration rate of 60 mL/min per 1.73 m2. Elevated hs‐TnT was associated with LVH and diastolic dysfunction at baseline in an adjusted model but was not associated with systolic dysfunction. These associations remained significant for both estimated glomerular filtration rate subgroups. Receiver operating characteristic curve analysis showed that hs‐TnT as a continuous variable exhibited fair significance for detection of LVH (area under the curve: 0.689) and diastolic dysfunction (area under the curve: 0.744). Multivariable analysis showed that higher hs‐TnT levels at baseline were related to development of LVH but not diastolic dysfunction (n=864).
Conclusions
In CKD patients, hs‐TnT is strongly associated with alterations of left ventricular structure and diastolic dysfunction for both estimated glomerular filtration rate strata. Baseline hs‐TnT levels are predictive of new LVH on follow‐up.
Keywords: chronic kidney disease, diastolic dysfunction, left ventricular hypertrophy, troponin T
Subject Categories: Cardiovascular Disease, Biomarkers, Nephrology and Kidney
Clinical Perspective
What Is New?
In patients with chronic kidney disease, high‐sensitivity troponin T (TnT) is strongly associated with alterations of left ventricular structure and diastolic dysfunction, regardless of estimated glomerular filtration rate strata.
Baseline levels of high‐sensitivity TnT are predictive of new left ventricular hypertrophy on follow‐up.
What Are the Clinical Implications?
This research suggests that a high‐sensitivity TnT assay could be a useful targeted strategy for risk stratification of patients who may need further cardiac evaluation, regardless of renal function.
Further studies are needed to confirm the ideal cutoff values for high‐sensitivity TnT in chronic kidney disease.
Introduction
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD). It is important to identify risk factors for prevention of cardiovascular disease in patients with CKD to facilitate treatment in the asymptomatic phase. Although pathologic changes in cardiac structure including left ventricular hypertrophy (LVH) may affect cardiovascular mortality,1, 2 they do not produce definite cardiac symptoms. For these reasons, physicians and researchers have invested considerable effort into identifying biomarkers related to subclinical changes in cardiac structure.
The conventional cardiac troponin T (TnT) assay has become a widely used biomarker for diagnosing acute coronary syndrome.3 Troponin assays have continued to evolve, and a high‐sensitivity TnT (hs‐TnT) assay with detection limits 10 to 100 times lower than conventional assays is now available. Importantly, hs‐TnT can be detected in asymptomatic patients with no history of cardiovascular disease.
Chronically elevated levels of hs‐TnT were recently shown to be associated with chronic subclinical myocardial damage in the general population, including left ventricular (LV) structure abnormalities.4, 5, 6 However, patients with CKD have persistently elevated TnT levels compared with those with normal renal function.7 In addition, chronic structural and functional abnormalities of the heart are common among CKD patients.8 Because hs‐TnT is easier to obtain in an outpatient setting and is relatively low in cost compared with a traditional echocardiogram, there is an important and clinically relevant need to define cardiac structural and functional correlates of hs‐TnT levels.9 The ability to interpret elevated troponin levels in CKD patients is critical, as their elevation may predict clinical and subclinical cardiac injury in patients with or without CKD.
To better understand the implication of elevated hs‐TnT level in CKD patients across a broad range of estimated glomerular filtration rates (eGFRs), we investigated the association between hs‐TnT and LV structure and function based on 2 different eGFR strata in the KNOW‐CKD (Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease), which comprises patients with both mild and severe renal dysfunction.
Methods
This study was performed using the database extracted from KNOW‐CKD, a Korean multicenter prospective cohort study that enrolled patients with non–dialysis‐dependent CKD (stages 1–5) from April 2011 to February 2016. The detailed design and methods for the KNOW‐CKD cohort have been published previously.10, 11 The study protocol was approved by the institutional review board at each participating clinical center. The study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol summary is registered at ClinicalTrials.gov under accession number NCT01630486. Written informed consent was obtained from all participants at the time of enrollment. Because of ethical issues and data protection regulations, data that support the findings of the present study cannot be made publicly available.
Briefly, a total of 2238 patients were enrolled in the KNOW‐CKD study. We excluded participants who, at enrollment, (1) did not have an hs‐TnT measurement at our central laboratory, (2) did not have an LV mass index measurement, or (3) had been diagnosed with heart failure. Finally, a total of 2017 patients were included in our cross‐sectional analysis (Figure 1). Patients who completed follow‐up transthoracic echocardiography after 4 years were included in an analysis of association between baseline hs‐TnT and changes in development of cardiac structure and function (n=864, 42.8% of total participants).
Figure 1.
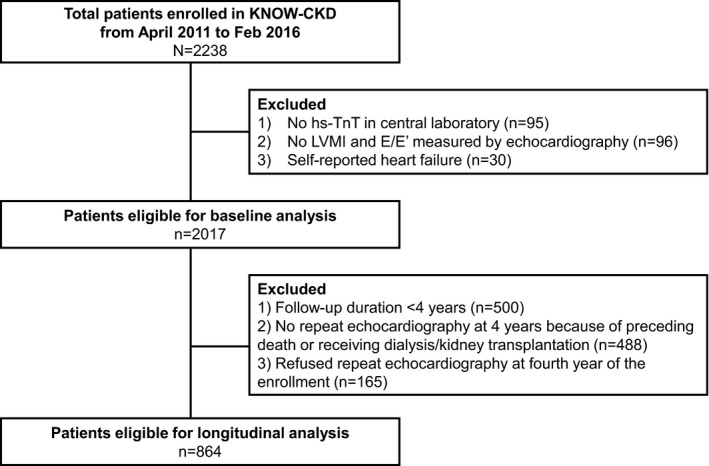
Study flow. hs‐TnT indicates high‐sensitivity cardiac troponin T (TnT); KNOW‐CKD, Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease; LVMI, left ventricular mass index.
All covariates analyzed in this study were collected at the time of enrollment. The primary predictor for echocardiographic parameters was the hs‐TnT level, which was measured using a highly sensitive electrochemiluminescence immunoassay on an Elecsys 2010, which has an analytical measurement range of 3 to 10 000 pg/mL. We investigated additional parameters including age; sex; body mass index; comorbidities including hypertension, diabetes mellitus, and coronary artery disease; hemoglobin; lipid profile; high‐sensitivity CRP (C‐reactive protein); and eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation with creatinine.12 Smoking status was categorized as current, ex‐, or never smoker.
The primary outcome was LVH and systolic and diastolic dysfunction. Two‐dimensional echocardiography was conducted at enrollment and during the fourth year of follow‐up. LV mass was calculated using the formula 0.8×{1.04 [(LVIDd+PWTd+SWTd)3−(LVIDd)3]}+0.6 g, where LVIDd refers to LV end‐diastolic internal dimension and PWTd and SWTd are posterior wall thickness at end diastole and septal wall thickness at end diastole, respectively.13 LV mass was indexed to height (m2.7) because LV mass indexed to body surface area is problematic in that weight is affected by volume overload in CKD.13, 14, 15 LVH was defined as an LV mass/height ≥47 g/m2.7 in women and ≥50 g/m2.7 in men. Relative wall thickness (RWT) was calculated as twice the posterior wall thickness/LV internal linear dimension in diastole. RWT values >0.42 were considered to be increased.13 LV mass and RWT were used to classify LV geometry: normal (no LVH and RWT ≤0.42), concentric remodeling (no LVH and RWT >0.42), eccentric hypertrophy (LVH and RWT ≤0.42), and concentric hypertrophy (LVH and RWT >0.42). LV systolic dysfunction was defined as an ejection fraction <50%.16 Diastolic dysfunction was defined as E/e′ measured at the medial annulus >15.17
Continuous variables are presented as mean±SD. Categorical variables are presented as proportion and frequency. We used 1‐way ANOVA for comparison of continuous variables and the χ2 test for categorical variables.
Patients were stratified into 4 groups according to hs‐TnT quartiles. We utilized multivariable logistic regression analysis to evaluate the independent association between hs‐TnT and outcomes of this study. A univariate analysis for each variable was conducted primarily, and the results were used to select the covariates included in the multivariate analysis based on P<0.05. Although some variables did not show statistical significance, variables of clinical importance were included based on the judgment of the researchers.
We stratified participants into 2 subgroups based on eGFR ≥60 or <60 mL/min per 1.73 m2 to evaluate the modifying effect of renal impairment on hs‐TnT. The eGFR strata did not show an interaction with the hs‐TnT level. P values for interactions between eGFR strata and hs‐TnT were 0.07, 0.55, and 0.41 for LVH, systolic dysfunction, and diastolic dysfunction, respectively. We carried out additional analyses to investigate whether hs‐TnT can be used as a screening test to detect structural and functional abnormalities. Receiver operating characteristic (ROC) curve analysis was performed for determination of area under the curve. Optimal cutoff concentrations for hs‐TnT were defined as points of the ROC curves by the Youden method.18 In addition, we conducted multivariate ROC analysis adjusted to covariables selected in the previous logistic regression, and the areas under the curve were compared according to renal function. Pairwise comparisons among the areas under the curve were made using the Delong method.19
A 2‐tailed P<0.05 was used as the cutoff for statistical significance. All statistical analyses were performed using R (v3.5.2; R Foundation for Statistical Computing) and SAS (v9.4; SAS Institute).
Results
The overall distribution of hs‐TnT was skewed to the right, and we observed an inverse relationship between hs‐TnT and eGFR (Figure S1). The mean participant age was 53.5±12.2 years, and 1229 (60.9%) patients were men (Table 1). The median hs‐TnT level was 10.0 pg/mL (interquartile range: 6.0–16.0 pg/mL), and the mean eGFR was 53.6±30.9 mL/min per 1.73 m2. Participants with higher hs‐TnT levels were older and more likely to be male and tended to have lower eGFRs and higher systolic blood pressures. In addition, higher hs‐TnT concentrations indicated higher percentages of patients diagnosed with diabetic or hypertensive nephropathy.
Table 1.
Baseline Characteristics of Participants According to hs‐TnT Level
| Total (N=2017) | TnT Category (pg/mL) | P Value | P for Trend | ||||
|---|---|---|---|---|---|---|---|
| ≤6.0 (n=517) | >6.0–10.0 (n=579) | >10.0–16.0 (n=436) | >16.0 (n=485) | ||||
| Age, y | 53.5±12.2 | 46.0±10.6 | 51.6±11.4 | 58.5±10.9 | 59.1±10.9 | <0.001 | <0.001 |
| Sex | <0.001 | <0.001 | |||||
| Female | 788 (39.1) | 288 (55.7) | 223 (38.5) | 155 (35.6) | 122 (25.2) | ||
| Male | 1229 (60.9) | 229 (44.3) | 356 (61.5) | 281 (64.4) | 363 (74.8) | ||
| BMI, kg/m2 | 24.6±3.4 | 24.0±3.5 | 24.6±3.2 | 24.7±3.3 | 25.1±3.4 | <0.001 | <0.001 |
| Blood pressure | |||||||
| SBP, mm Hg | 127.9±16.2 | 125.0±14.1 | 126.3±14.8 | 127.7±15.6 | 133.2±18.9 | <0.001 | <0.001 |
| DBP, mm Hg | 77.1±11.1 | 78.3±10.5 | 77.5±10.3 | 75.7±10.7 | 76.5±12.7 | 0.002 | <0.001 |
| MAP, mm Hg | 94.0±11.6 | 93.8±10.9 | 93.8±10.9 | 93.1±11.0 | 95.4±13.6 | 0.02 | 0.20 |
| Current smoker | 325 (16.1) | 79 (15.3) | 97 (16.8) | 76 (17.4) | 73 (15.1) | <0.001 | |
| DM | 673 (33.4) | 60 (11.6) | 112 (19.3) | 172 (39.4) | 329 (67.8) | <0.001 | <0.001 |
| Hypertension | 1937 (96.0) | 466 (90.1) | 563 (97.2) | 432 (99.1) | 476 (98.1) | <0.001 | <0.001 |
| CAD | 102 (5.1) | 4 (0.8) | 18 (3.1) | 28 (6.4) | 52 (10.7) | <0.001 | <0.001 |
| Cause of CKD | <0.001 | 0.10 | |||||
| Diabetic nephropathy | 466 (23.1) | 21 (4.1) | 52 (9.0) | 122 (28.0) | 271 (55.9) | ||
| Glomerulonephritis | 720 (35.7) | 232 (44.9) | 283 (48.9) | 128 (29.4) | 77 (15.9) | ||
| Hypertensive nephropathy | 368 (18.2) | 56 (10.8) | 100 (17.3) | 118 (27.1) | 94 (19.4) | ||
| ADPKD | 344 (17.1) | 181 (35.0) | 115 (19.9) | 35 (8.0) | 13 (2.7) | ||
| Unknown | 119 (5.9) | 27 (5.2) | 29 (5.0) | 33 (7.6) | 30 (6.2) | ||
| eGFR, mL/min/1.73 m2 | 53.6±30.9 | 80.9±28.9 | 58.6±26.2 | 40.9±21.7 | 29.8±17.3 | <0.001 | <0.001 |
| CKD stage | <0.001 | <0.001 | |||||
| Stage 1 | 335 (16.6) | 217 (42.0) | 91 (15.7) | 21 (4.8) | 6 (1.2) | ||
| Stage 2 | 385 (19.1) | 162 (31.3) | 152 (26.3) | 46 (10.6) | 25 (5.2) | ||
| Stage 3a | 328 (16.3) | 72 (13.9) | 131 (22.6) | 85 (19.5) | 40 (8.2) | ||
| Stage 3b | 428 (21.2) | 50 (9.7) | 132 (22.8) | 131 (30.0) | 115 (23.7) | ||
| Stage 4 | 417 (20.7) | 14 (2.7) | 64 (11.1) | 120 (27.5) | 219 (45.2) | ||
| Stage 5 | 124 (6.1) | 2 (0.4) | 9 (1.6) | 33 (7.6) | 80 (16.5) | ||
| Laboratory findings | |||||||
| CRP, mg/dL | 2.0±5.2 | 1.5±4.3 | 1.9±4.6 | 2.2±5.5 | 2.5±6.5 | 0.01 | <0.001 |
| HDL, mg/dL | 49.3±15.5 | 54.2±15.9 | 50.7±14.7 | 46.3±13.4 | 45.0±15.9 | <0.001 | <0.001 |
| Triglyceride, mg/dL | 157.4±97.9 | 144.6±89.9 | 155.4±98.4 | 163.2±93.2 | 167.7±107.5 | 0.001 | |
| Hemoglobin, g/dL | 12.9±2.0 | 13.6±1.7 | 13.4±1.9 | 12.5±2.0 | 11.7±1.9 | <0.001 | <0.001 |
| Urine protein/creatinine ratio (g/g, IQR) | 0.5 (0.1–1.5) | 0.3 (0.07–0.7) | 0.3 (0.1–0.9) | 0.5 (0.2–1.5) | 1.4 (0.5–3.4) | <0.001 | <0.001 |
| Urine albumin/creatinine ratio (mg/mg, IQR) | 347.7 (76.8–1052.1) | 167.5 (27.9–530.0) | 242.8 (59.1–665.7) | 366.4 (83.3–1063.2) | 997.0 (350.8–2378.9) | <0.001 | <0.001 |
| Data from echocardiography | |||||||
| LVMI, g/m2.7 | 41.9±11.5 | 36.6±9.0 | 39.7±9.2 | 43.8±10.7 | 48.3±13.5 | <0.001 | <0.001 |
| LVH | 483 (23.9) | 56 (10.8) | 92 (15.9) | 129 (29.6) | 206 (42.5) | <0.001 | <0.001 |
| LV geometry | <0.001 | <0.001 | |||||
| Normal | 1237 (61.3) | 392 (75.8) | 400 (69.1) | 246 (56.4) | 199 (41.0) | ||
| Concentric LVH | 245 (12.1) | 38 (7.4) | 58 (10.0) | 55 (12.6) | 94 (19.4) | ||
| Concentric remodeling | 294 (14.6) | 57 (11.0) | 87 (15.0) | 70 (16.1) | 80 (16.5) | ||
| Eccentric LVH | 236 (11.7) | 29 (5.6) | 33 (5.7) | 64 (14.7) | 110 (22.7) | ||
| E/E′ | 9.9±3.7 | 8.3±2.4 | 9.2±3.0 | 10.4±3.8 | 11.9±4.4 | <0.001 | <0.001 |
| Ejection fraction, % | 64.2±5.9 | 63.8±5.6 | 64.7±5.6 | 64.1±5.8 | 64.2±6.7 | 0.08 | 0.04 |
| Systolic dysfunction | 21 (1.0) | 2 (0.4) | 4 (0.7) | 4 (0.9) | 11 (2.3) | <0.001 | <0.001 |
| Diastolic dysfunction | 167 (8.3) | 8 (1.5) | 26 (4.5) | 47 (10.8) | 86 (17.7) | <0.001 | <0.001 |
Data are shown as mean±SD or n (%). ADPKD indicates autosomal dominant polycystic kidney disease; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CRP, C‐reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hs‐TnT, high‐sensitivity troponin T; IQR, interquartile range; LV, left ventricular; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; MAP, mean arterial pressure; SBP, systolic blood pressure.
Overall, 483 patients (23.9%) had LVH on echocardiography. LV mass index increased gradually across the hs‐TnT quartile groups. The proportion of concentric LVH, concentric remodeling, and eccentric LVH all gradually increased across the quartiles of cardiac TnT concentration (Figure 2). There were 21 patients (1.0%) with systolic dysfunction and 167 patients (8.3%) with diastolic dysfunction. The proportions of both LV dysfunctions were higher in patients with higher hs‐TnT levels than in those with lower levels.
Figure 2.
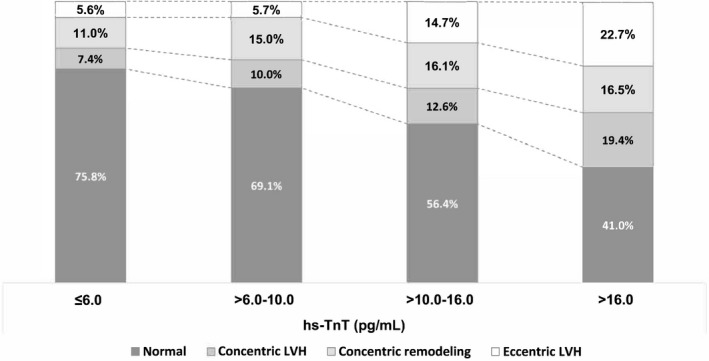
Elevated hs‐TnT levels are associated with LVH in patients with chronic kidney disease. With increasing quartiles of hs‐TnT, the prevalence of concentric and eccentric LVH and concentric remodeling increases. hs‐TnT indicates high‐sensitivity cardiac troponin T; LVH, left ventricular hypertrophy;
In the univariate analysis, LVH and systolic and diastolic dysfunction were related to increment of hs‐TnT (Table S1). Table 2 summarizes the independent association between hs‐TnT and LVH or diastolic dysfunction with multivariable analysis. At baseline, the 2 highest quartiles of hs‐TnT were found to have an odds ratio >2 for LVH in the fully adjusted model (P for trend <0.001; Figure 3). The eGFR strata did not show a statistically significant interaction for the hs‐TnT level in both LVH and diastolic dysfunction (P=0.07 and P=0.41, respectively, for interactions). The highest quartiles of hs‐TnT were significantly associated with LVH in both eGFR strata, suggesting the results were independent of renal function in multivariable analysis. No significant association between hs‐TnT and systolic dysfunction was identified in multivariable analysis. Higher hs‐TnT concentrations were independently associated with diastolic dysfunction in our fully adjusted model (P<0.001 for trend). In a stratified analysis according to eGFR, diastolic dysfunction continued to show a significant relationship with the highest hs‐TnT range for both eGFR strata.
Table 2.
Association Between hs‐TnT and LV Structure and Functional Abnormalities According to eGFR
| hs‐TnT (pg/mL) | Odds of LVHa | P Value | Odds of Systolic Dysfunctionb | P Value | Odds of Diastolic Dysfunctionc | P Value | |
|---|---|---|---|---|---|---|---|
| Total patients | ≤6.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| >6.0–10.0 | 1.17 (0.78–1.78) | 0.46 | 1.06 (0.20–7.87) | 0.95 | 2.60 (1.11–6.84) | 0.03 | |
| >10.0–16.0 | 2.02 (1.29–3.19) | 0.002 | 1.23 (0.20–10.07) | 0.83 | 4.77 (2.012–12.80) | <0.001 | |
| >16.0 | 3.17 (1.94–5.25) | <0.001 | 2.20 (0.41–17.50) | 0.39 | 7.71 (3.10–21.49) | <0.001 | |
| eGFR ≥60 mL/min/1.73 m2 | ≤6.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| >6.0–10.0 | 1.35 (0.73–2.49) | 0.33 | 1.08 (0.08–14.00) | 0.95 | 1.64 (0.44–6.8548) | 0.46 | |
| >10.0–16.0 | 1.86 (0.77–4.35) | 0.15 | 3.91 (0.27–55.70) | 0.29 | 1.72 (0.358–8.59) | 0.49 | |
| >16.0 | 14.75 (4.72–48.37) | <0.001 | 6.02 (0.38–94.00) | 0.18 | 12.41 (2.42–70.27) | 0.003 | |
| eGFR <60 mL/min/1.73 m2 | ≤6.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| >6.0–10.0 | 0.87 (0.488–1.60) | 0.65 | 1.05 (0.13–21.79) | 0.96 | 1.97 (0.62–8.73) | 0.29 | |
| >10.0–16.0 | 1.54 (0.865–2.83) | 0.15 | 0.86 (0.09–18.87) | 0.91 | 3.96 (1.31–17.23) | 0.03 | |
| >16.0 | 2.17 (1.174–4.12) | 0.01 | 1.63 (0.22–33.79) | 0.68 | 6.24 (2.00–27.67) | 0.005 |
eGFR indicates estimated glomerular filtration rate; hs‐TnT, high‐sensitivity troponin T; LV, left ventricular; LVH, left ventricular hypertrophy.
LVH: adjusted for age, sex, mean arterial pressure, diabetes mellitus, hypertension, coronary artery disease, chronic kidney disease stage, body mass index, high‐density lipoprotein, triglyceride, and hemoglobin.
Systolic dysfunction: adjusted for age, sex, coronary artery disease, chronic kidney disease stage.
Diastolic dysfunction: adjusted for age, sex, diabetes mellitus, hypertension, chronic kidney disease stage, body mass index, high‐density lipoprotein, triglyceride, C‐reactive protein, and smoking history.
Figure 3.
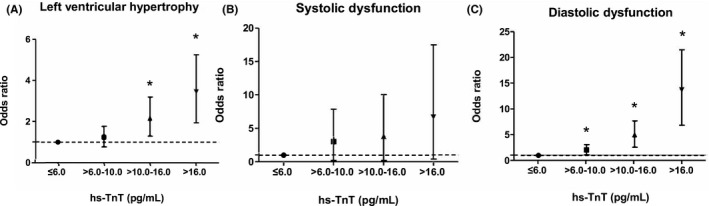
Association between hs‐TnT and left ventricular structure and function at baseline echocardiography. A, Left ventricular hypertrophy: adjusted for age, sex, mean arterial pressure, DM, hypertension, coronary artery disease, CKD stage, mean arterial pressure, BMI, HDL (high‐density lipoprotein), triglyceride, hemoglobin. B, Systolic dysfunction: adjusted for age, sex, coronary artery disease, CKD stage. C, Diastolic dysfunction: adjusted for age, sex, DM, hypertension, CKD stage, BMI, HDL, triglyceride, CRP (C‐reactive protein), smoking history. BMI indicates body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; hs‐TnT, high‐sensitivity troponin T. * indicates P<0.05.
We next evaluated whether hs‐TnT as a continuous value can be used as a screening test for LVH and LV function in a CKD population (Table 3). Levels of hs‐TnT exhibited fair significance for detection of each outcome. By ROC analysis, the optimized hs‐TnT cutoff value for LVH was 9 pg/mL for patients with an eGFR ≥60 mL/min per 1.73 m2 and 15 pg/mL for those with an eGFR <60 mL/min per 1.73 m2, respectively. With respect to diastolic dysfunction, the optimized hs‐TnT cutoff values were 9 and 14 pg/mL for patients with eGFRs ≥60 and <60 mL/min per 1.73 m2, respectively. When comparing the multivariable ROC curves with other covariates for the 2 eGFR strata, the differences between the 2 curves were statistically significant for both LVH and diastolic dysfunction (Figure S2).
Table 3.
hs‐TnT as a Single Diagnostic Test for LVH and Diastolic Dysfunction
| AUC (95% CI) | Optimal Cutoff (pg/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | |
|---|---|---|---|---|---|---|---|---|
| LVH | ||||||||
| Total participants | 0.69 (0.66–0.72) | 10 | 69.4 | 61.8 | 13.5 | 83.6 | 1.8 | 0.5 |
| eGFR ≥60 mL/min/1.73 m2 | 0.63 (0.585–0.68) | 9 | 38.7 | 81.9 | 11.7 | 74.5 | 2.3 | 0.8 |
| eGFR <60 mL/min/1.73 m2 | 0.64 (0.61–0.68) | 15 | 56.2 | 63.9 | 24.0 | 57.2 | 1.4 | 0.5 |
| Systolic dysfunction | ||||||||
| Total participants | 0.69 (0.58–0.80) | 16 | 52.4 | 76.3 | 0.7 | 97.7 | 2.2 | 0.4 |
| eGFR ≥60 mL/min/1.73 m2 | 0.78 (0.56–1.00) | 8 | 75.0 | 76.1 | 0.2 | 98.3 | 3.1 | 0.3 |
| eGFR <60 mL/min/1.73 m2 | 0.64 (0.49–0.79) | 31 | 41.2 | 88.5 | 0.9 | 95.5 | 3.6 | 0.7 |
| Diastolic dysfunction | ||||||||
| Total participants | 0.74 (0.71–0.78) | 12 | 79.5 | 57.3 | 3.5 | 83.6 | 2.2 | 0.4 |
| eGFR ≥60 mL/min/1.73 m2 | 0.71 (0.63–0.79) | 9 | 54.3 | 80.2 | 2.4 | 90.3 | 2.2 | 0.4 |
| eGFR <60 mL/min/1.73 m2 | 0.70 (0.65–0.74) | 14 | 64.2 | 60.3 | 4.9 | 80.6 | 1.8 | 0.5 |
AUC indicates area under the curve; eGFR, estimated glomerular filtration rate; hs‐TnT, high‐sensitivity cardiac troponin T; LR, likelihood ratio; LVH, left ventricular hypertrophy; NPV, negative predictive value; PPV, positive predictive value.
Among all 2017 enrolled patients, 864 patients underwent follow‐up echocardiography after 4 years of enrollment. Excluding patients who were previously diagnosed with LVH at baseline echocardiography, 86 (12.3%) of 698 patients developed new LVH. Among 828 patients who did not have diastolic dysfunction at baseline, 44 patients (5.3%) developed new diastolic dysfunction at 4 years. Only 7 patients were diagnosed with new systolic dysfunction. The percentage of patients who developed new LVH and diastolic dysfunction increased with elevation of hs‐TnT levels. In terms of LV geometry at 4 years, the results were similar to the baseline in that each proportion of concentric LVH, concentric remodeling, and eccentric LVH increased across the quartiles of cardiac TnT concentration (Figure S3). Finally, multivariable analysis showed that increased hs‐TnT was related to development of LVH (P<0.001 for trend; Figure 4A) but not to diastolic dysfunction (P=0.262 for trend; Figure 4B).
Figure 4.
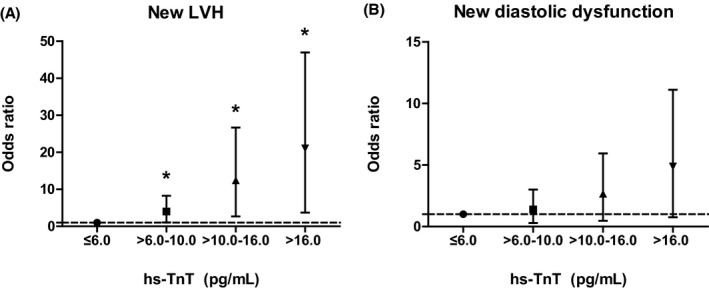
The association between hs‐TnT and follow‐up echocardiography. A, New LVH: adjusted for age, sex, mean arterial pressure, DM, hypertension, coronary artery disease, CKD stage, BMI, HDL (high‐density lipoprotein), triglyceride, hemoglobin. B, New diastolic dysfunction: adjusted to age, sex, DM, hypertension, CKD stage, BMI, HDL, triglyceride, CRP (C‐reactive protein), smoking history. BMI indicates body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; hs‐TnT, high‐sensitivity troponin T; LVH, left ventricular hypertrophy. * indicates P<0.05.
Discussion
We investigated the relationship between hs‐TnT and LV structure and function in CKD patients with mild to severe renal dysfunction. Consistent with previous reports,20, 21, 22 increased TnT was independently associated with LVH. To our knowledge, this study is the first regarding the association between diastolic dysfunction and hs‐TnT in predialysis CKD patients. Considering previous reports that hs‐TnT was elevated with lower renal function,7, 23, 24 we conducted further analyses in which we stratified patients into 2 subgroups based on renal function as determined by eGFR. Elevated hs‐TnT was significantly associated with LVH and diastolic dysfunction in both eGFR strata.
As techniques for measuring biomarkers have become more sensitive, it has become easier to detect TnT using a highly sensitive assay. Importantly, growing evidence suggests that elevated hs‐TnT levels in the chronic setting with no cardiac symptoms and without acute coronary syndrome may indicate the presence of chronic subclinical myocardial damage.4, 5, 6 However, a previous retrospective observational study showed that a reduced eGFR is associated with a gradual increase in hs‐TnT at the individual and population levels, meaning there is an inverse relationship between hs‐TnT and eGFR.23 Reduced renal clearance of TnT in CKD patients has been suggested as an explanation7, 25; however, there is controversy. To date, it has not been clear whether chronic elevation of cardiac troponin levels in CKD patients is related to decreased renal clearance or increased cardiac release. Considering the relationship between renal function and hs‐TnT, it remains unclear whether detection of hs‐TnT in asymptomatic patients is meaningful for identifying chronic myocardial damage. Consequently, one of the greatest advantages of the present study was our assessment of the association between hs‐TnT levels and cardiac structural and functional abnormalities across various stages of CKD, from relatively preserved renal function to severe renal dysfunction.
There seemed to be some differences in hs‐TnT concentrations among various causes of CKD. However, as we reported previously elsewhere,11 diabetic or hypertensive nephropathy subgroups included more participants with lower eGFR at advanced CKD stages. Therefore, the difference in troponin levels among various CKD causes could be attributed to differences in eGFR distribution among various CKD causes.
Explanations of the relationship between hs‐TnT and LV structural and functional abnormalities remain speculative. LVH is caused by LV pressure and/or volume overload in an attempt to maintain wall stress, which in turn leads to myocyte death that may be further exacerbated by decreased coronary perfusion, and uremia.21, 26 In particular, uremia is an important factors for changing myocardial structure regardless of pressure and volume overload in both animal and human studies.27, 28 Renal impairment provokes accumulation of hypertrophic substances related to uremia such as EDN1 (endothelin 1), parathyroid hormone, TNF‐α (tumor necrosis factor α), and IL‐1α (interleukin 1α) and IL‐6.29 In addition, LVH may lead to release of hs‐TnT in hypertrophied hearts, possibly reflecting changes in cell membrane permeability and myocardial protein turnover.20, 30
Systolic and diastolic dysfunction is associated with increased hs‐TnT in the both general population4, 5 and in patients with end‐stage renal disease.31, 32, 33 Studies on non–dialysis‐dependent CKD patients are rare, thus the results of the present study may help enrich our understanding of the relationship among hs‐TnT, CKD, and cardiovascular disease. Indeed, we observed an independent association between the highest quartile hs‐TnT group and diastolic dysfunction independent of renal function. In contrast to the CRIC (Chronic Renal Insufficiency Cohort) study,20 an association between hs‐TnT and systolic dysfunction was not identified in our study; however, we did find that hs‐TnT was associated with diastolic dysfunction. At enrollment, participants who were previously diagnosed with congestive heart failure (New York Heart Association functional class III or IV) were excluded in the KNOW‐CKD cohort. Consequently, it was difficult to perform additional analyses for systolic dysfunction.
The results of our study suggest continuous hs‐TnT as a possible screening test for LVH and systolic and diastolic dysfunction, regardless of renal function, before additional costly evaluation such as echocardiographic study. Specifically, statistically significant differences were noted between the areas under the curve according to renal function for all outcomes. We carried out additional analyses to identify optimal values of hs‐TnT and found that the ideal cutoff values for hs‐TnT were higher in patients with a lower eGFR for LVH and systolic and diastolic dysfunction. These findings suggest that hs‐TnT might be affected by renal function. However, because of the low prevalence of LVH and LV systolic or diastolic dysfunction among our study participants, the positive predictive values were very low. Positive and negative likelihood ratios were calculated to reduce the influence of low prevalence (Table 3), and the results suggested that hs‐TnT alone has some limitation as a definitive screening test for LVH and LV dysfunction. However, considering that echocardiography is an expensive test, the present study showed the possibility of hs‐TnT as a simple and cost‐effective test for risk stratification for patients who need further echocardiographic evaluation.
To elucidate the temporal relationship between hs‐TnT and LV structural and functional abnormalities, we conducted additional analyses using follow‐up data. Follow‐up echocardiography at 4 years was offered to all participants, regardless of cardiac symptoms. Those who died or developed end‐stage renal disease before 4 years or who did not reach 4‐year follow‐up did not undergo repeated echocardiography. Higher hs‐TnT levels were associated only with development of LVH and not with diastolic dysfunction. However, because the number of participants who underwent follow‐up echocardiography was small, further analyses are warranted to elucidate the causal relationships between hs‐TnT and changes in cardiac structure and function over time.
Several limitations of this study deserve attention. First, we depended on a single hs‐TnT measurement and thus were unable to perform additional analyses of changes of hs‐TnT levels over time. Second, this study was conducted exclusively in an Asian population without preexisting severe heart failure. In addition, relatively few patients had systolic dysfunction, and this limited the statistical power. Moreover, echocardiographic interpretations could be prone to poor interrater reliability depending on the settings, and the echocardiography was conducted by different cardiologists. Nonetheless, we shared the same formula for calculating LV mass index and uniform echocardiographic measurement of E/e′ at the medial annulus of the mitral valve. Last, echocardiographic follow‐up was conducted in <50% of the study participants for various reasons, including dialysis initiation, death, and dropout.
In summary, hs‐TnT was strongly associated with alterations of LV structure and diastolic dysfunction. These tendencies persisted in a stratified analysis according to eGFR. Although the predictive power was limited in the low eGFR group, hs‐TnT measurement from CKD patients appears to be a useful targeted strategy that can be used for risk stratification of patients who may need further cardiac evaluation, regardless of renal function. The results of a 4‐year follow‐up showed that increased cardiac TnT was associated with development of LVH. If such an hs‐TnT screening test were incorporated, patients identified at higher risk should be encouraged to perform further evaluations regularly or to undergo more targeted interventions to reduce their risk of LVH and diastolic dysfunction. Further studies are needed to confirm the ideal cutoff values of hs‐TnT in various CKD populations, and such efforts will help early detection of cardiac structural and functional abnormalities and improve cardiovascular outcomes for patients with CKD.
Author Contributions
Study design: Oh and Ahn; acquisition of data: K.‐B. Lee, Chae, Sung, S. W. Kim, Ahn, and Oh; data analysis: Kang, Ryu, J. Kim, J. Lee; writing the article: Kang; review, revision, and final approval: Oh. All authors were involved in drafting this article and/or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication.
Sources of Funding
This study was supported by the research program funded by the Korea Center for Disease Control and Prevention (KCDC; 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, and 2016E3300200). The study was supervised by the Chronic Kidney Disease Advisory Committee, composed of members from the KCDC and the Korean Society of Nephrology (ClinicalTrials.gov, NCT01630486). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Table S1. Univariate Analysis of High‐Sensitivity TnT (Troponin T) and Left Ventricular Hypertrophy and Systolic and Diastolic Dysfunction
Figure S1. Distributions of high‐sensitivity TnT (troponin T) in the KNOW‐CKD (Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease) cohort.
Figure S2. Receiver operating characteristic curve and area under the curve after adjustment of covariables according to renal function.
Figure S3. Elevated high‐sensitivity TnT (troponin T) is associated with development of left ventricular hypertrophy in patients with chronic kidney disease at the fourth year after enrollment.
Acknowledgments
We are grateful to Yeji Hong, who helped with statistical analysis in this study.
(J Am Heart Assoc. 2019;8:e013357 DOI: 10.1161/JAHA.119.013357.)
References
- 1. Cioffi G, Tarantini L, Frizzi R, Stefenelli C, Russo TE, Selmi A, Toller C, Furlanello F, de Simone G. Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens. 2011;29:565–573. [DOI] [PubMed] [Google Scholar]
- 2. Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351. [DOI] [PubMed] [Google Scholar]
- 3. Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. 2011;58:1819–1824. [DOI] [PubMed] [Google Scholar]
- 4. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. deFilippi C, Seliger SL, Kelley W, Duh SH, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J. Interpreting cardiac troponin results from high‐sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem. 2012;58:1342–1351. [DOI] [PubMed] [Google Scholar]
- 8. Matsushita K, Ballew SH, Coresh J. Influence of chronic kidney disease on cardiac structure and function. Curr Hypertens Rep. 2015;17:581. [DOI] [PubMed] [Google Scholar]
- 9. Hyunjeong B, Jung Ah K, So‐Yeon C, Yeon‐Sil D, Eun‐Hee J, Jung In K, Jung Ho D, Sung Chul C, Jung Eun L, Wooseong H, Dae Joong K, Ha‐Young O, Seung W. Original article: brain natriuretic peptide (BNP), N‐terminal pro‐BNP (NT‐proBNP) and cardiac troponin T (cTnT) as markers of cardiac diseases in stable hemodialysis patients. Kidney Res Clin Pract. 2007;26:212–219. Available at: http://www.krcp-ksn.org/journal/view.html?uid=2948&vmd=Full. [Google Scholar]
- 10. Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, Chung W, Hwang YH, Kim SW, Kim YH, Kang SW, Park BJ, Lee J, Ahn C. KNOW‐CKD (Korean cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. 2014;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang E, Han M, Kim H, Park SK, Lee J, Hyun YY, Kim Y‐S, Chung W, Kim HJ, Oh YK, Ahn C, Oh K‐H. Baseline general characteristics of the Korean chronic kidney disease: report from the Korean cohort study for outcomes in patients with chronic kidney disease (KNOW‐CKD). J Korean Med Sci. 2017;32:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 14. de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. [DOI] [PubMed] [Google Scholar]
- 15. Glassock RJ, Pecoits‐Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4(suppl 1):S79–S91. [DOI] [PubMed] [Google Scholar]
- 16. Mahadevan G, Davis RC, Frenneaux MP, Hobbs FD, Lip GY, Sanderson JE, Davies MK. Left ventricular ejection fraction: are the revised cut‐off points for defining systolic dysfunction sufficiently evidence based? Heart. 2008;94:426–428. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 18. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 19. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20. Mishra RK, Li Y, DeFilippi C, Fischer MJ, Yang W, Keane M, Chen J, He J, Kallem R, Horwitz EJ, Rafey M, Raj DS, Go AS, Shlipak MG. Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013;61:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallamaci F, Zoccali C, Parlongo S, Tripepi G, Benedetto FA, Cutrupi S, Bonanno G, Fatuzzo P, Rapisarda F, Seminara G, Stancanelli B, Bellanuova I, Cataliotti A, Malatino LS. Troponin is related to left ventricular mass and predicts all‐cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2002;40:68–75. [DOI] [PubMed] [Google Scholar]
- 22. Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, Dries DL, Go AS, He J, Kusek JW, Lash JP, Raj D, Rosas S, Wolf M, Zhang X, Shlipak MG, Feldman HI. High‐sensitivity troponin T and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and risk of incident heart failure in patients with CKD: the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2015;26:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung JZ, Dallas Jones GR. Effect of renal function on serum cardiac troponin T—population and individual effects. Clin Biochem. 2015;48:807–810. [DOI] [PubMed] [Google Scholar]
- 24. Khaled Abdul‐Aziz A, Wahda Mohammed A‐A. Prognostic performance of combined use of high‐sensitivity troponin T and creatine kinase MB isoenzyme in high cardiovascular risk patients with end‐stage renal disease. Kidney Res Clin Pract. 2017;36:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen‐Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–25. [DOI] [PubMed] [Google Scholar]
- 26. Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M. Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Fail. 2013;19:E40–E45. [DOI] [PubMed] [Google Scholar]
- 27. McMahon AC, Greenwald SE, Dodd SM, Hurst MJ, Raine AE. Prolonged calcium transients and myocardial remodelling in early experimental uraemia. Nephrol Dial Transplant. 2002;17:759–764. [DOI] [PubMed] [Google Scholar]
- 28. Wolf WC, Yoshida H, Agata J, Chao L, Chao J. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int. 2000;58:730–739. [DOI] [PubMed] [Google Scholar]
- 29. Winchester JF, Audia PF. Extracorporeal strategies for the removal of middle molecules. Semin Dial. 2006;19:110–114. [DOI] [PubMed] [Google Scholar]
- 30. Francis GS, Tang WH. Cardiac troponins in renal insufficiency and other non‐ischemic cardiac conditions. Prog Cardiovasc Dis. 2004;47:196–206. [DOI] [PubMed] [Google Scholar]
- 31. deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, Christenson R, Uretsky B, Smiley M, Gold J, Muniz H, Badalamenti J, Herzog C, Henrich W. Cardiac troponin T and C‐reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long‐term hemodialysis. JAMA. 2003;290:353–359. [DOI] [PubMed] [Google Scholar]
- 32. Sharma R, Gaze DC, Pellerin D, Mehta RL, Gregson H, Streather CP, Collinson PO, Brecker SJ. Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T. Heart. 2006;92:804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cerasola G, Nardi E, Palermo A, Mule G, Cottone S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol. 2011;24:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Analysis of High‐Sensitivity TnT (Troponin T) and Left Ventricular Hypertrophy and Systolic and Diastolic Dysfunction
Figure S1. Distributions of high‐sensitivity TnT (troponin T) in the KNOW‐CKD (Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease) cohort.
Figure S2. Receiver operating characteristic curve and area under the curve after adjustment of covariables according to renal function.
Figure S3. Elevated high‐sensitivity TnT (troponin T) is associated with development of left ventricular hypertrophy in patients with chronic kidney disease at the fourth year after enrollment.


