Figure 5.
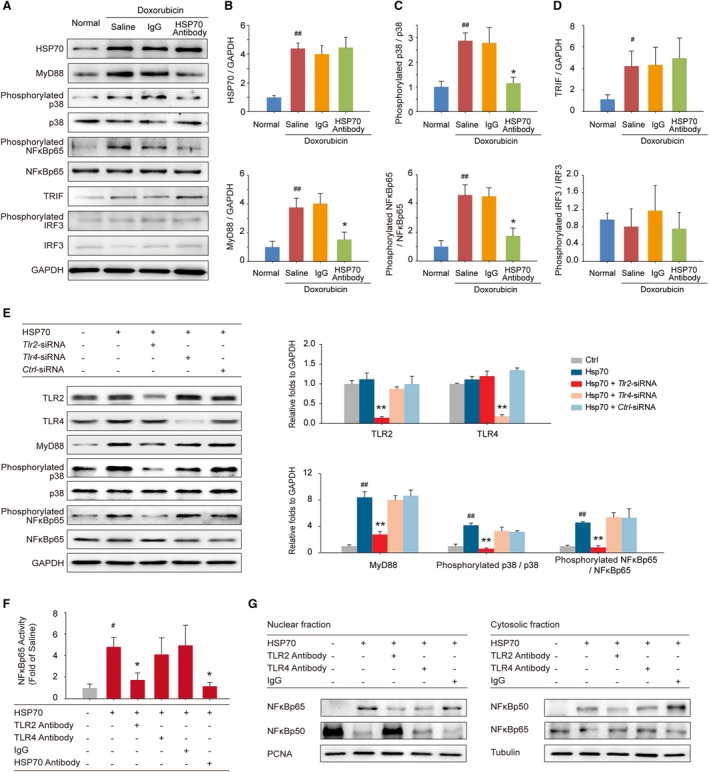
Extracellular HSP (heat shock protein) 70 activates nuclear factor (NF)‐κBp65 and modulates the distribution of the NF‐κB p50 and p65 subunits in a toll‐like receptor 2 (TLR2)–dependent manner. A, Expressions of HSP70, MyD88, phosphorylated p38, p38, phosphorylated NF‐κBp65, NF‐κBp65, toll like receptor adaptor molecule 1 (TRIF), phosphorylated interferon regulatory factor 3 (IRF3), and IRF3 in the mouse myocardium were detected by Western blot analysis. Neutralizing extracellular HSP70 inhibited the MyD88–p38–NF‐κB pathway (B and C) but did not affect the TRIF‐IRF3 pathway (D). Representative immunoblots and the ratio of the indicated protein to GAPDH are presented. # P<0.05, ## P<0.01, compared with normal mice; *P<0.05, **P<0.01, compared with doxorubicin‐treated mice. E, Tlr2–small interfering RNA (siRNA)–, Tlr4‐siRNA–, or Ctrl‐siRNA–transfected H9C2 cells were treated with 100 ng/mL HSP70 recombinant protein for 24 hours; and the TLR2, TLR4, MyD88, phosphorylated p38, p38, phosphorylated NF‐κBp65, and NF‐κBp65 were detected by Western blot analysis. Representative immunoblots and the ratio of the indicated protein to GAPDH are presented. ## P<0.01, compared with untreated cells; **P<0.01, compared with HSP70‐treated cells. F and G, TLR2 antibody, TLR4 antibody, or IgG preincubated H9C2 cells were treated with HSP70 recombinant protein. F, The phosphorylation of NF‐κBp65 in cell homogenates was detected by ELISA analysis. # P<0.05, compared with untreated cells; *P<0.05, compared with HSP70‐treated cells. G, Nuclear and cytosolic extracts were subjected to Western blot analysis with antibodies to the NF‐κB subunits p50 and p65. PCNA and tubulin were used as internal controls for the nuclear and cytosolic fractions, respectively. Representative immunoblots of 3 independent assays are presented.
