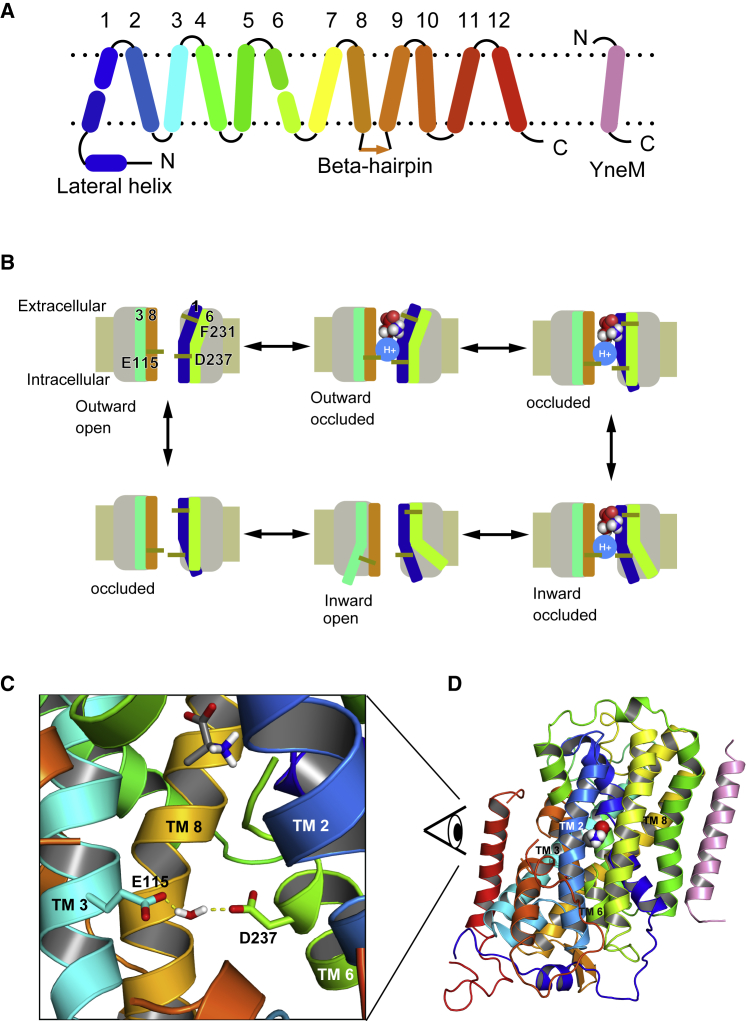
Figure 1.
Structure and mechanism of GkApcT. (A) Shown is a schematic diagram of the GkApcT fold, in which the first five helices are an inverted repeat of the next five helices, similar to LeuT. The YneM helix (pink) is an additional subunit present in the crystal structure and included in the simulations presented here. (B) Mechanisms of the proton coupled transport cycle are as follows: outward-open state, outward-occluded state with substrate and proton bound, occluded state, inward-occluded state with substrate and proton ready to leave, inward-open state, and apo occluded state. (C) GkApcT in the inward-occluded state in complex with alanine is shown. The substrate alanine is drawn using the space fill representation. The two key residues gating the intracellular gate are E115 from TM 3 (aqua stick) and D237 from TM6 (green). The water molecule is not directly observed in the crystal but appears during the molecular dynamics simulations. (D) Shown is a zoomed-out view of GkApcT contextualizing the view in (C). To see this figure in color, go online.