Abstract
Introduction
The definitive treatment for severe heart failure is transplantation. However, only a small number of heart transplants are performed each year due to donor shortages. Therefore, novel treatment approaches based on artificial organs or regenerative therapy are being developed as alternatives. We have developed a technology known as cell sheet-based tissue engineering that enables the fabrication of functional three-dimensional (3D) tissue. Here, we report a new technique for engineering human cardiac tissue with perfusable blood vessels. Our method involved the layering of cardiac cell sheets derived from human induced pluripotent stem cells (hiPSCs) on a vascular bed derived from porcine small intestinal tissue.
Methods
For the vascular bed, a segment of porcine small intestine was harvested together with a branch of the superior mesenteric artery and a branch of the superior mesenteric vein. The small intestinal tissue was incised longitudinally, and the mucosa was resected. Human cardiomyocytes derived from hiPSCs were co-cultured with endothelial cells and fibroblasts on a temperature-responsive dish and harvested as a cardiac cell sheet. A triple-layer of cardiac cell sheets was placed onto the vascular bed, and the resulting construct was subjected to perfusion culture in a bioreactor system.
Results
The cardiac tissue on the vascular bed pulsated spontaneously and synchronously after one day of perfusion culture. Electrophysiological recordings revealed regular action potentials and a beating rate of 105 ± 13/min (n = 8). Furthermore, immunostaining experiments detected partial connection of the blood vessels between the vascular bed and cardiac cell sheets.
Conclusions
We succeeded in engineering spontaneously beating 3D cardiac tissue in vitro using human cardiac cell sheets and a vascular bed derived from porcine small intestine. Further development of this method might allow the fabrication of functional cardiac tissue that could be used in the treatment of severe heart failure.
Keywords: Cardiac cell sheet, Vascular bed, Perfusion culture, hiPSCs, Angiogenesis
Abbreviations: 3D, three-dimensional; bFGF, basic fibroblast growth factor; DMEM, Dulbecco's Modified Eagle Medium; ECM, extracellular matrix; GFP, green fluorescent protein; HE, hematoxylin/eosin; hiPSC, human induced pluripotent stem cells; HUVECs, human umbilical vein endothelial cells; NHDFs, normal human dermal fibroblasts; PERV, porcine endogenous retrovirus; VEGF, vascular endothelial growth factor
1. Introduction
The definitive treatment for end-stage heart failure is transplantation, but the number of heart transplants performed each year is limited due to a shortage of donors [1,2]. Therefore, novel regenerative therapies are being developed based on the injection of cells or transplantation of bioengineered tissue. Although cell injection is a straightforward technique that can improve cardiac function or promote cardiomyocyte regeneration [[3], [4], [5], [6], [7]], the benefits are thought to arise from paracrine effects exerted by the injected cells, most of which die after a few days due to anoikis (a form of programmed cell death) and ischemia [8].
The creation of thick specimens of beating cardiac tissue for transplantation into a failing heart might overcome some of the limitations of the cell injection method. Scaffold-based tissue engineering generates three-dimensional (3D) tissues on a biodegradable polymer scaffold [[9], [10], [11], [12]]. However, the tissues fabricated with this technique have a limited thickness because they lack a functional vascular network to provide oxygenation, nutrient delivery and elimination of waste products. An important goal in the field of tissue engineering is the generation of vascularized tissue, and several recent studies have reported potential vascularization techniques [[15], [16], [17], [18], [19]].
Our research group has developed a method of fabricating vascularized cardiac tissue using cell sheet-based tissue engineering. Rat neonatal cardiac cell sheets created by this technique exhibit morphological and electrical connections and beat spontaneously and synchronously [13,14]. Furthermore, we have successfully fabricated 1-mm thick rat cardiac tissue in vivo by sequentially layering cardiac cell sheets onto the subcutaneous tissue of a recipient [20].
The development of an in vitro method of generating vascularized cardiac tissue would be an important step toward the clinical application of cell sheet-based tissue engineering in the management of severe heart failure. Vukadinovic-Nikolic et al. reported that bioartificial rat heart tissue could be fabricated by combining a gel-based cardiac construct with decellularized small intestinal submucosa [21]. We have successfully engineered a 200-μm-thick specimen of cardiac tissue in vitro with the aid of a vascular bed derived from rat femoral (skeletal) muscle, which was ‘prevascularized’ in vivo before use to ensure that it had a rich microvascular network. Rat neonatal cardiac cells were co-cultured with endothelial cells to form cell sheets, and a triple-layered sheet was implanted onto the vascular bed every 3 days during perfusion culture in a custom-made bioreactor system. The resulting cardiac tissue was transplanted into a rat by anastomosis of the tissue's artery and vein with blood vessels in the animal. Importantly, the pulsation and vascular structure of the transplanted tissue were maintained at 2 weeks after transplantation, indicating that the tissue was still viable [22].
The clinical application of cell sheet-based tissue engineering relies on the fabrication of human cardiac tissue. Thick specimens of human cardiac tissue derived from human induced pluripotent stem cells (hiPSCs) have been generated in rat subcutaneous tissue using a multi-step cell sheet transplantation technique [23]. However, no previous studies have fabricated thick vascularized human cardiac tissue in vitro by layering tissue-engineered cell sheets on a large vascular bed.
The aim of this study was to bioengineer human cardiac tissue in vitro using hiPSC-derived cardiac cell sheets and a large vascular bed obtained from an animal. We report the successful isolation of a large-scale vascular bed from the pig small intestine and the creation of engineered human cardiac tissue in vitro by perfusion culture of hiPSC-derived cardiac cell sheets on the isolated vascular bed. The technique described in this study may have the potential to be developed into a new clinical therapy for diseases such as heart failure.
2. Methods
All animal experiments were approved by the Ethics Committee for Animal Experimentation of Tokyo Women's Medical University and performed according to the Guidelines of Tokyo Women's Medical University on Animal Use.
2.1. Vascular bed fabrication
Candidate vascular beds for perfusion culture were generated from small intestine or omentum and their accompanying blood vessels, which were obtained from male pigs (15 kg; Sanesu Breeding, Chiba, Japan). Medetomidine (Domitor; 40 μg/kg; Nippon Zenyaku Kogyo, Fukushima, Japan) and midazolam (Dormicum; 0.25 mg/kg; Astellas Pharma, Tokyo, Japan) were administered 10–15 min before the induction of general anesthesia with 2.5% sevoflurane (Mylan; Canonsburg, PA, USA). A 10 cm length of small intestine (together with a branch of the superior mesenteric artery and a branch of the superior mesenteric vein) or the omentum (together with a branch of the gastroepiploic artery and a branch of the gastroepiploic vein) was resected using an electric knife. The small intestine was incised longitudinally along the side opposite the mesentery, and its mucosa was resected with a fine knife. The blood vessels of each specimen were cannulated using polyurethane tubes so that the tissue could be perfused with culture medium or other agents such as black ink (Fig. 1). Once the tissues had been harvested, the pig was sacrificed by intravenous administration of potassium chloride under anesthesia.
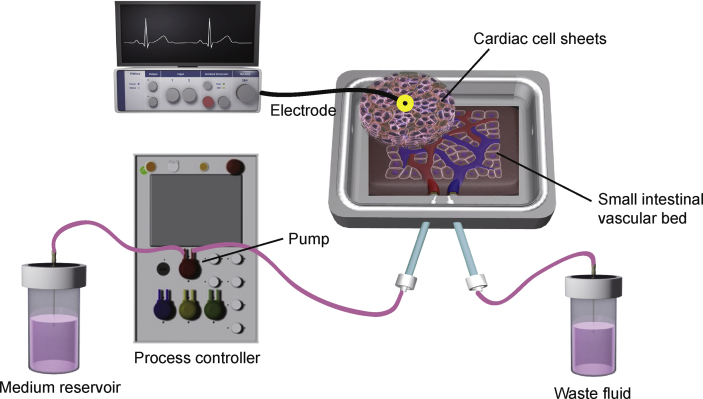
Fig. 1.
Scheme illustrating the in vitro fabrication of three-dimensional vascularized cardiac tissue. Cardiac cells derived from human induced pluripotent stem cells were co-cultured with endothelial cells (ECs) as a monolayer sheet on a temperature-responsive culture dish. Three cardiac cell sheets were harvested and stacked to create three-dimensional cardiac tissue. The triple-layered cardiac cell sheet was placed onto a vascular bed derived from porcine small intestine, which had been cut open and had its mucosa resected. The vascular bed was perfused via branches of the superior mesenteric artery and vein in a custom-made bioreactor system. The bioreactor system consisted of a tissue culture chamber and delivery pump, which included a flow transmitter, pressure transmitter and temperature transmitter. The perfusion of culture medium via the artery was driven by the pump, and waste fluid was drained from the vein. Surface action potential were recorded from the cardiac cell sheet and amplified by bioelectric amplifiers. After several days of perfusion culture, the co-cultured ECs were able to connect to the small vessels of the vascular bed to create a well-organized endothelial cell network between the vascular bed and triple-layered cardiac cell sheet.
2.2. Bioreactor set-up and tissue perfusion culture
The custom-made bioreactor (Tokai Hit, Shizuoka, Japan) was a one-pass system consisting of a microprocessor-controlled delivery pump, a custom-made tissue culture chamber, a flow transmitter and a pressure transmitter. The temperature transmitter was used to maintain the temperature of the tissue culture chamber at 37 °C. The vascular bed was placed in the tissue culture chamber and perfused with Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum at a pressure of 40 mmHg (flow, 200–500 μL/min). Medium was perfused via the inlet tube connected to the small intestinal artery, and the volume of medium leaving the outlet was measured by a digital weighing scale. The temperature, arterial pressure and volume of perfused medium were recorded during perfusion culture (Fig. 1). Neovascularization was enhanced by the addition of 16 ng/mL basic fibroblast growth factor (bFGF) to the culture medium.
2.3. Cardiomyocytes derived from hiPSCs and fabrication of cell sheets
hiPSCs (201B7, RIKEN, Tsukuba, Japan) [24] expressing the puromycin resistance gene under the control of the alpha myosin heavy chain promoter [25] were cultured in the bioreactor system to induce their differentiation into cardiomyocytes, as previously reported [23,26]. After differentiation, the cardiomyocytes were cultured on a normal dish for 3 days and purified by the addition of puromycin (200 ng/mL) for 4 days. To induce formation of a vascular network, purified cardiomyocytes were co-cultured with green fluorescent protein (GFP)-positive human umbilical vein endothelial cells (HUVECs; Angio-Proteomie, Boston, MA, USA) and adult normal human dermal fibroblasts (NHDFs; Lonza, Basel, Switzerland) at a ratio of 5:1:5. A total of 1.0 × 106 cells were plated on a 35-mm temperature-responsive culture dish (UpCell; CellSeed, Tokyo, Japan) and cultured in DMEM containing 10% fetal bovine serum (37 °C, 5% CO2). After 3 days, the co-cultured cardiac cells were maintained at 20 °C for 30 min to harvest them as a monolayer sheet. NHDFs or cardiomyocytes were also co-cultured (under similar conditions) with HUVECs only at a ratio of 10:1, and the endothelial cell networks of the fabricated cell sheets were compared. For immunohistochemistry, the cells were cultured for 3 days and then fixed with 4% paraformaldehyde for 5 min. The cells were incubated overnight at 4 °C with a 1/500 dilution of rabbit anti-cardiac troponin T primary antibody (Abcam, Cambridge, UK) and a 1/500 dilution of anti-hCD31-PE primary antibody (R&D systems, Minneapolis, MN, USA). After washing with PBS, the samples were incubated for 1 h at room temperature with a 1/200 dilution of Alexa-Fluor-568-conjugated goat anti-rabbit IgG secondary antibody (Thermo Fisher Scientific).
2.4. Cell sheet layering and implantation onto the vascular bed
A cardiac cell sheet was harvested from a temperature-responsive culture dish and suspended in culture medium. The culture medium was gently aspirated, and the detached cell sheet was placed on a culture dish as the first layer. The cell sheet was incubated at 37 °C for 1 h to reattach it to the surface of the culture dish. Next, a second cell sheet together with culture medium was aspirated onto the tip of a pipette, transferred onto the upper surface of the first cell sheet and allowed to adhere after aspiration of the culture medium. After incubation of the double-layered cell sheet at 37 °C for 1 h, a third cell sheet was added in the same manner to produce a triple-layered construct. Triple-layered cardiac cell sheets were placed onto the vascular bed, and the resulting tissue construct was subjected to perfusion culture in the bioreactor system.
2.5. Histological analysis
After perfusion culture, the fabricated tissues (porcine small intestinal vascular bed and implanted cardiac cell sheets) were fixed with 4% paraformaldehyde. Tissue sections were stained with hematoxylin/eosin (HE) or Azan trichrome using conventional methods. To detect cardiomyocytes and endothelial cells, deparaffinized sections were incubated overnight at 4 °C with a 1/100 dilution of anti-cardiac troponin T monoclonal primary antibody (Thermo Fisher Scientific) and a 1/10 dilution of rabbit anti-CD31 polyclonal primary antibody (Thermo Fisher Scientific). Specimens were subsequently treated with 1/200 dilutions of Alexa-Fluor-568-conjugated goat anti-mouse IgG (Thermo Fisher Scientific) and Alexa-Fluor-488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific) secondary antibodies for 1 h at room temperature. To detect GFP-HUVECs after perfusion culture, paraffin-embedded sections were incubated overnight at 4 °C with a 1/100 dilution of rabbit anti-CD31 polyclonal primary antibody (Thermo Fisher Scientific) and a 1/200 dilution of anti-GFP monoclonal primary antibody (Thermo Fisher Scientific). Specimens were then treated with 1/200 dilutions of Alexa-Fluor-568-conjugated goat anti-rabbit IgG (Invitrogen) and Alexa-Fluor-488-conjugated donkey anti-mouse IgG (Thermo Fisher primary) secondary antibodies for 2 h at room temperature. Cell nuclei were counterstained by 6-diamidino-2-phenylindole (Invitrogen) for 5 min at 4 °C. The sections were observed using a confocal laser-scanning microscope (FV1200 IX83; Olympus, Tokyo, Japan).
2.6. Electrophysiological analysis
The bioreactor was opened, and two needle electrodes (29G in diameter; ADInstruments, New South Wales, Australia) were placed over the cardiac tissue. Surface action potentials were amplified (UA102 amplifier, Unique Medical, Tokyo, Japan) and recorded using a data acquisition system (ML870 PowerLab 8/30; ADInstruments).
2.7. Measurement of blood capillary density
To quantify blood vessel formation in the omentum and small intestine, three random locations were selected from each Azan-stained tissue cross-section, and the number of capillaries were counted in each field from light microscopic images obtained at a magnification of ×100 (n = 3 per group).
2.8. Statistical analysis
All data are expressed as the mean ± standard deviation. An unpaired Student's t-test was performed to compare values between two groups. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Vascular bed selection and preparation for perfusion culture
Porcine omentum was the first candidate considered for use as a vascular bed and was harvested with its artery and vein. Unfortunately, perfusion culture of the omentum was difficult because the diameter of the vein was too small for cannulation. The proximal side of the omentum was then dissected until larger vessels were reached that were suitable for cannulation and perfusion. However, the porcine omentum contained little fat or connective tissue (Fig. 2a), which resulted in handling difficulties. Additionally, perfusion with black ink resulted in the staining of only a narrow area of tissue (Fig. 2b; n = 2), suggesting only limited perfusion of the omentum. Staining with Azan trichrome indicated that the omentum was not rich in extracellular matrix or blood vessels (Fig. 2c).
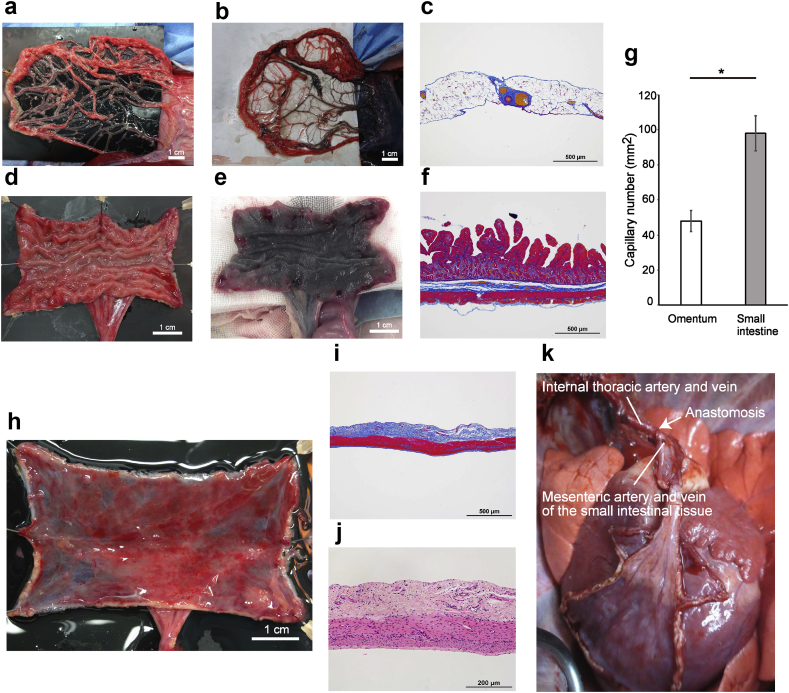
Fig. 2.
Fabrication of a vascular bed using the porcine small intestine. (a) Porcine omentum was the first candidate for the vascular bed, but this tissue was poor in fat and extracellular matrix and thus difficult to handle. (b) Black ink perfused into the gastroepiploic artery stained only a few areas of the porcine omentum. (c) Azan trichrome staining of the porcine omentum revealed that this tissue was not rich in extracellular matrix or blood vessels. (d) The porcine small intestine was incised longitudinally after harvesting to create a planar vascular bed. (e) Black ink perfused via a branch of the superior mesenteric artery stained almost the entire region of the porcine small intestine, demonstrating that this tissue had a rich vascular network. (f) Azan trichrome staining of the porcine small intestine showed the presence of a rich extracellular matrix surrounding the submucosa. (g) Porcine small intestine had significantly more capillaries than porcine omentum (p < 0.05). The number of capillaries was counted in three sections from each of three samples. The analysis was performed independently by two different technicians. *p < 0.05. (h) The porcine small intestine was incised longitudinally and opened out, and its mucosa was resected with a fine knife. (i) Azan trichrome staining of the vascular bed created from porcine small intestine revealed adequate resection of the mucosa and preservation of the extracellular matrix and small vessels (before perfusion culture). (j) Hematoxylin-eosin staining of the vascular bed created from porcine small intestine demonstrated that the tissue had not been damaged by mucosal resection and that nuclei were detected throughout the remaining tissue (before perfusion culture). (k) The harvested small intestine was successfully transplanted into another pig by anastomosis of the branches of the superior mesenteric artery and vein (supplying the small intestinal tissue) to the left internal thoracic artery and vein of the recipient animal.
Porcine small intestine was evaluated as an alternative candidate for use as a vascular bed because it has dense microvasculature and could be used as a planar vascular bed after longitudinal incision. Therefore, a segment of porcine small intestine was harvested together with a branch of the superior mesenteric artery and a branch of the superior mesenteric vein. Notably, the small intestine preparation contained considerably more tissue (such as mucosa and submucosa; Fig. 2d and e) than the omentum, and injected black ink reached almost all regions of the small intestine (Fig. 2e; n = 2). Azan trichrome staining revealed more connective tissue (blue area) in the small intestine than in the omentum (Fig. 2c and f). Moreover, the small intestine had a significantly larger number of capillaries than the omentum (p < 0.05; n = 3; Fig. 2g). The above findings suggested that porcine small intestine was better suited for use as a vascular bed than omentum. Therefore, the small intestine was used for subsequent experiments.
Next, the small intestine was incised longitudinally, and its mucosa was resected with a surgical knife so that the vascular network of the submucosa could be utilized as a planar vascular bed for perfusion culture (Fig. 2h). Histological analysis confirmed that the mucosa had been resected and the submucosa preserved without damage to the tissue (Fig. 2i). Cell nuclei were present throughout the remaining tissue (Fig. 2j; before perfusion culture, day 0). The viability of the harvested small intestine was demonstrated by its successful transplantation into another pig, which was achieved by anastomosing the branches of the superior mesenteric artery and vein of the small intestinal tissue with the left internal thoracic artery and vein, respectively, of the recipient pig (Fig. 2k; n = 1).
3.2. Fabrication of cardiac cell sheets with an endothelial cell network
Spontaneously beating cardiomyocytes were derived from hiPSCs using a previously reported procedure [23,25,26]. When HUVECs were co-cultured with NHDFs, an endothelial cell network was observed (Fig. 3a). However, when cardiomyocytes were co-cultured with GFP-positive HUVECs on a temperature-responsive culture dish to fabricate cardiac cell sheets, a network of CD31-positive cells (i.e. an endothelial cell network) was not detected (Fig. 3b). When cardiomyocytes were co-cultured with both GFP-positive HUVECs and NHDFs at a ratio of 5:1:5, an endothelial cell network was observed within the cardiac cells (Fig. 3c). The cardiomyocytes exhibited spontaneous beating 3 days later, at which time they were harvested as a monolayer sheet (Fig. 3d).
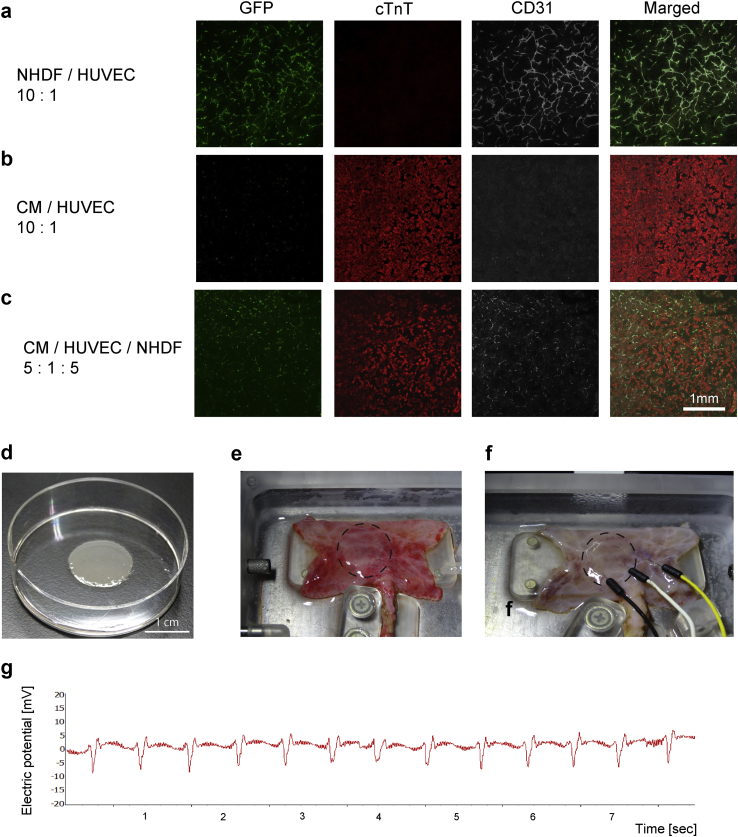
Fig. 3.
Cardiac cell sheet implantation. (a) An endothelial cell network (evaluated from the expression of CD31, an endothelial cell marker) was clearly detected following the co-culture of green fluorescent protein (GFP)-expressing human umbilical vein endothelial cells (HUVECs) with adult normal human dermal fibroblasts (NHDFs) at a ratio of 10:1. cTnT: cardiac troponin T. (b) An endothelial cell network was not detected after the co-culture of cardiomyocytes derived from human induced pluripotent stem cells (hiPSCs) and GFP-positive HUVECs. CM: cardiomyocytes. (c) An endothelial cell network was created when cardiomyocytes were co-cultured with GFP-positive HUVECs and NHDFs at a ratio of 5:1:5. (d) Cardiomyocytes derived from hiPSCs were co-cultured with GFP-positive HUVECs in a temperature-responsive dish and harvested as a monolayer sheet. The harvested sheet shrank to approximately one-third of the dish diameter. (e) Three harvested cardiac cell sheets were layered, and this triple-layered cell sheet (broken circle) was placed onto a vascular bed created from porcine small intestine (day 0). The fabricated tissue construct was then subjected to perfusion culture for 6 days. (f) Needle electrodes were used to record surface action potentials from the implanted cardiac cell sheet (broken circle) after 6 days of perfusion culture. (g) Representative trace showing surface action potentials recorded from the implanted cardiac cell sheet after 6 days of perfusion culture. The implanted cardiac cell sheet was observed to beat spontaneously with a regular rhythm.
3.3. Perfusion culture of the small intestinal vascular bed and cardiac cell sheets using a bioreactor system
A triple layer of cardiac cell sheets was implanted onto the porcine small intestine (submucosal side), and the resulting construct was subjected to perfusion culture at a perfusion pressure of 40 mmHg (Fig. 3e and f). Synchronized spontaneous beating of the cardiac cell sheets was first observed on post-implantation day 1 and was maintained up to day 6. Moreover, the cardiac tissue showed no evidence of necrosis. Electrophysiological recordings (Fig. 3f and g) revealed regular action potentials occurring at a rate of 105 ± 13/min (n = 8), which agreed with the beating rate measured by direct observation of the contracting tissue.
3.4. In vitro neovascularization of the tissue construct during perfusion culture
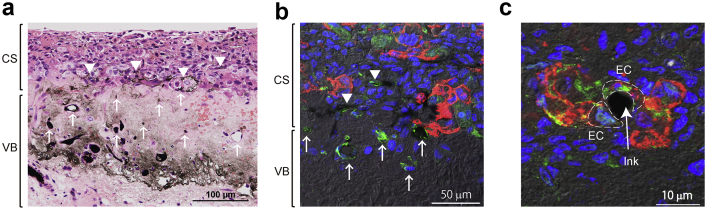
The structures of the porcine small intestine and implanted cardiac cell sheets were well maintained even after 6-days of perfusion culture, and nuclei were detected in both the vascular bed and implanted cell sheets. The submucosa of the porcine small intestine and the cardiomyocytes of the implanted cell sheets were readily identifiable, and no abnormal spaces or tissues were found between the cardiac cell sheets and small intestine. After 6 days of perfusion culture, black ink administered via the small intestinal artery was detected in the implanted cardiac cell sheets and small intestine (Fig. 4a; n = 5). Immunostaining revealed that both cardiomyocytes (red cells) and endothelial cells (green cells) from the implanted sheets were still present after 6 days of perfusion culture (Fig. 4b), and GFP-HUVECs were confirmed as the origin of the endothelial cells in the cell sheet (Fig. 4c). Immunostaining also identified black ink surrounded by CD31-positive cells (Fig. 4b and c). These findings reveal that the small intestine and implanted cardiac cell sheets remained viable throughout a 6-day period of perfusion culture, with successful attachment of the cell sheets to the vascular bed and the formation of connections between small vessels in the two tissues.
Fig. 4.
Vascularization between the implanted cardiac cell sheet and vascular bed. (a) After perfusion culture of the vascular bed and cardiac cell sheet for 6 days, black ink was perfused via the artery of the vascular bed. Hematoxylin-eosin staining revealed that the tissue had retained its viability and that the cardiac cell sheet was attached to the porcine small intestine. Black ink was occasionally detected within the implanted cardiac cell sheet (arrows). CS: cardiac cell sheet; VB: vascular bed. (b) Immunostaining for CD31 (green: endothelial cells, ECs) and troponin T (red: cardiomyocytes from the cardiac cell sheet) revealed that cardiomyocytes were still present in the cell sheet after 6 days of perfusion culture and that ECs formed tubular structures that resembled microvessels. Perfused black ink was observed inside the microvessels of the cell sheet (arrows). (c) Immunostaining for CD31 (red: all ECs) and green fluorescent protein (GFP)-positive human umbilical vein endothelial cells (green: co-cultured ECs in the cell sheet) demonstrated that ECs originating from the cardiac cell sheet had formed microvessels. Black ink perfused via the vascular bed was detected in a vessel formed by ECs from the cell sheet (arrow).
4. Discussion
The present study describes the successful fabrication of 3D cardiac tissue in vitro. Cardiac cell sheets with an endothelial cell network were created using cardiomyocytes derived from hiPSCs, GFP-positive HUVECs and NHDFs. The cell sheets were implanted onto a large vascular bed made from porcine small intestinal tissue. Spontaneous, regular beating of the triple-layered cardiac cell sheet was observed after 1 day of perfusion culture, and connections between the endothelial cell network of the cardiac sheet and small vessels of the intestinal tissue were detected after 6 days.
The omentum was initially thought to be an appropriate candidate for a vascular bed because it has an abundant blood supply, angiogenic ability and a high level of growth factor production [27]. Moreover, human omentum has been used clinically during surgery to cover tissue defects, prevent infections and avert peptic ulcer perforation [[28], [29], [30], [31]]. However, we found the omentum to be unsuitable for use as a vascular bed for two reasons. First, the omental vein was too narrow for drainage of the perfusate during perfusion culture, and the use of a larger vessel exposed by dissection failed to achieve widespread perfusion of the omentum. Second, the porcine omentum is not rich in fat, connective tissue or ECM, which makes it difficult to manipulate. Furthermore, these tissue components play roles in angiogenesis, adherence to surrounding tissue and production of growth factors [32].
The porcine small intestine was selected as an alternative candidate for a vascular bed because it is rich in blood vessels, submucosal connective tissue and ECM. Importantly, at least three segments of small intestine supplied by branches of the superior mesenteric artery and vein could be harvested from one pig. Black ink stained the entire segment of small intestine, demonstrating that the whole tissue was being perfused. Thus, porcine small intestine appears well suited for use as a vascular bed for tissue engineering.
Transplantation of the tissue was achieved by anastomosis of its vessels with those of the recipient (Supplemental Video). Although xenotransplant immunogenicity limits the clinical usefulness of our current construct, this potentially could be overcome by decellularizing the animal tissue and recellularizing it with human endothelial cells. The successful decellularization and recellularization of a whole rat heart has been reported [33], and others have also attempted to decellularize whole organs [[34], [35], [36], [37]]. Decellularized tissue has been used as a scaffold for tissue engineering [38], although this approach has its limitations. Nevertheless, decellularized tissues or organs could be appropriate future candidates for vascular beds because the ECM, which remains after decellularization, is similar in structure and composition between species [39]. This might mitigate against adverse immune reactions after the placement of cell sheets and promote angiogenesis as well as cell migration, differentiation and proliferation [32]. Since it would be easier to recellularize only the vascular network with endothelial cells rather than the entire organ, one of our future objectives is to fabricate a decellularized vascular bed with a recellularized vascular network.
The following is the supplementary data related to this article:
1
Another problem associated with xenotransplantation is the possible transmission of porcine endogenous retrovirus (PERV), which is found in all pig strains and can infect human cells. However, the inactivation of PERV with a gene editing technique was recently reported [40], suggesting that porcine tissue could become a future source of bioartificial tissues or organs.
Cardiac cell sheets with an endothelial cell network produce high levels of growth factors such as vascular endothelial growth factor (VEGF), bFGF and hepatocyte growth factor and exhibit greater angiogenesis after transplantation than sheets without an endothelial cell network [16,41,42]. In this study, the cardiac cell sheets were fabricated using the best co-culture conditions currently available. However, an endothelial cell network was not created when cardiomyocytes were co-cultured with human endothelial cells alone, indicating that further research is required to optimize the fabrication of cardiac cell sheets with an endothelial cell network.
Immunostaining and black ink administration revealed only partial connections between the small blood vessels of the vascular bed and the endothelial cell network of the cardiac cell sheets. Complete angiogenesis would facilitate the fabrication of thicker specimens of cardiac tissue, but the reason why this did not occur in our fabricated tissue is unclear. The porcine small intestine has many microvessels (Fig. 2e), suggesting that more endothelial cells may be required in the cardiac cell sheet to facilitate angiogenesis. If so, different conditions would be needed to generate a richer vascular network in the cell sheet. The fabrication of completely vascularized cardiac tissue will require further experiments to establish the optimal co-culture conditions for the vascular network, for example by varying the bFGF concentration, VEGF level or duration of perfusion culture.
Previous studies have used the perfusion culture technique to engineer thick specimens of rat cardiac tissue with perfusable blood vessels [22], vascularize custom-made microcapillaries in a scaffold [17], vascularize fabricated 3D microcapillaries in an ‘AngioChip’ [18] and revascularize decellularized tissue with endothelial cells [19]. The present study used a custom-made bioreactor system for perfusion culture of hiPSC-derived cardiac cell sheets on a non-decellularized porcine small intestinal vascular bed. The fabricated tissue remained viable over a 6-day period, and partial connections were formed between the endothelial cell network of the cardiac sheets and the blood vessels of the small intestinal vascular bed. Of course, the conditions used for perfusion culture in the bioreactor system are not the same as those in vivo because physiological phenomena such as pressure from the local environment or body motion were not considered. In future, it may be necessary to take these other factors into consideration in order to fabricate more complex tissues or organs.
Allogeneic transplantation of the fabricated tissue construct onto the surface of the left ventricle of a pig was achieved by surgical anastomosis of the vessels. As a next step, we intend to transplant a thick section of fabricated cardiac tissue into a porcine model of myocardial infarction and evaluate the changes in left ventricular function. Thicker cardiac tissue will be created using a decellularized vascular bed and human cardiac cell sheets. If obstacles such as immunogenicity, sterility and scalability are successfully overcome, the transplantation of thick sections of bioengineered cardiac tissue could potentially become a new treatment option for severe heart failure. Furthermore, it should be possible to modify our technique to create other tissues or organs such as lung, liver or kidney.
5. Conclusions
We succeeded in fabricating a visibly beating 3D cardiac tissue in vitro using a porcine small intestinal vascular bed and cardiomyocytes derived from hiPSCs. We also identified the formation of partial connections between the small blood vessels of the vascular bed and the endothelial cell network of the cardiac cell sheets. The bioengineered cardiac tissue was transplanted into another pig and observed to beat spontaneously after reperfusion with blood. If issues of immunogenicity, sterility and scalability are successfully overcome, we believe that this technology has the potential to be developed into a method of generating thick sections of functional cardiac tissue for use in the treatment of severe heart failure.
Declaration of competing interest
Kazunori Sano is an employee of Tokai Hit Co., Ltd (Japan). Eiji Kobayashi is a medical adviser for Sysmex Corp. (Japan) and Regience Ltd. (Japan). Tatsuya Shimizu is a member of the scientific advisory board and a shareholder of CellSeed Inc. (Japan). Tokyo Women's Medical University received research funds from CellSeed Inc. Tatsuya Shimizu and Katsuhisa Matsuura are inventers of the bioreactor system for the differentiation and culture of pluripotent stem cells, the patent of which is held by Able Corp. (Japan) and Tokyo Women's Medical University (Cell culture apparatus and cell culture method using the same, US9574165B2). Tokyo Women's Medical University received a research grant from Tokaihit Co., Ltd, Japan.
Acknowledgments
This research was supported by the “Development of innovative manufacturing technology for three-dimensional tissues and organs based on cell sheet engineering” from the Japan Agency for Medical Research and Development (AMED, https://www.amed.go.jp/en/index.html; grant no. JP17he0702249) and JSPS KAKENHI Grant Number 19H04453. The authors thank OXMEDCOMMS (www.oxmedcomms.com) for writing assistance.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ. The registry of the international society for heart and lung transplantation: thirty-third adult heart transplantation report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1158–1169. doi: 10.1016/j.healun.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima N., Ono M., Saiki Y., Sawa Y., Nunoda S., Isobe M. Registry report on heart transplantation in Japan (June 2016) Circ J. 2017;81:298–303. doi: 10.1253/circj.CJ-16-0976. [DOI] [PubMed] [Google Scholar]
- 3.Chugh A.R., Beache G.M., Loughran J.H., Mewton N., Elmore J.B., Kajstura J. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126 doi: 10.1161/CIRCULATIONAHA.112.092627. S54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare J.M., Fishman J.E., Gerstenblith G., DiFede Velazquez D.L., Zambrano J.P., Suncion V.Y. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Menasché P., Hagège A.A., Scorsin M., Pouzet B., Desnos M., Duboc D. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 8.Robey T.E., Saiget M.K., Reinecke H., Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley C., Wong J.M., Fisher D.M., Khan W.S. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr Stem Cell Res Ther. 2013;8:243–252. doi: 10.2174/1574888x11308030009. [DOI] [PubMed] [Google Scholar]
- 10.Nemeno-Guanzon J.G., Lee S., Berg J.R., Jo Y.H., Yeo J.E., Nam B.M. Trends in tissue engineering for blood vessels. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/956345. 956345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuan R.S., Chen A.F., Klatt B.A. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–311. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildirimer L., Thanh N.T., Seifalian A.M. Skin regeneration scaffolds: a multimodal bottom-up approach. Trends Biotechnol. 2012;30:638–648. doi: 10.1016/j.tibtech.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40–e48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 15.Richardson T.P., Peters M.C., Ennett A.B., Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2011;11:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 16.Sekiya S., Shimizu T., Yamato M., Kikuchi A., Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341:573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 17.Chouinard J.A., Gagnon S., Couture M.G., Lévesque A., Vermetee P. Design and validation of a pulsatile perfusion bioreactor for 3D high cell density cultures. Biotechnol Bioeng. 2009;104:1215–1223. doi: 10.1002/bit.22477. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B., Montgomery M., Chamberlain M.D., Ogawa S., Korolj A., Pahnke A. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016;15:669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groeber F, Kahlig A, Loff S, Walles H, Hansmann J. A bioreactor system for interfacial culture and physiological perfusion of vascularized tissue equivalents. Biotechnol J. 2013;8:308–316. doi: 10.1002/biot.201200160. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T., Sekine H., Yang J., Isoi Y., Yamato M., Kikuchi A. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 21.Vukadinovic-Nikolic Z, Andrée B, Dorfman SE, Pflaum M, Horvath T, Lux M. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa. Tissue Eng Part A. 2014;20:799–809. doi: 10.1089/ten.TEA.2013.0184. [DOI] [PubMed] [Google Scholar]
- 22.Sekine H., Shimizu T., Sakaguchi K., Dobashi I., Wada M., Yamato M. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komae H, Sekine H, Dobashi I, Matsuura K, Ono M, Okano T. Three-dimensional functional human myocardial tissues fabricated from induced pluripotent stem cells. J Tissue Eng Regen Med. 2017;11:926–935. doi: 10.1002/term.1995. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Seta H., Matsuura K., Sekine H., Yamazaki K., Shimizu T. Tubular cardiac tissues derived from human induced pluripotent stem cells generate pulse pressure in vivo. Sci Rep. 2017;7:45499. doi: 10.1038/srep45499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuura K., Wada M., Shimizu T., Haraguchi Y., Sato F., Sugiyama K. Creation of human cardiac cell sheets using pluripotent stem cells. Biochem Biophys Res Commun. 2012;425:321–327. doi: 10.1016/j.bbrc.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 27.Porzionato A., Sfriso M.M., Macchi V., Rambaldo A., Lago G., Lancerotto L. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur J Histochem. 2013;57 doi: 10.4081/ejh.2013.e4. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spindler N., Etz C.D., Misfeld M., Josten C., Mohr F.W., Langer S. Omentum flap as a salvage procedure in deep sternal wound infection. Ther Clin Risk Manag. 2017;13:1077–1083. doi: 10.2147/TCRM.S134869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Wingerden J.J., Lapid O., Boonstra P.W., de Mol B.A. Muscle flaps or omental flap in the management of deep sternal wound infection. Interact Cardiovasc Thorac Surg. 2011;13:179–187. doi: 10.1510/icvts.2011.270652. [DOI] [PubMed] [Google Scholar]
- 30.Lin B.C., Liao C.H., Wang S.Y., Hwang T.L. Laparoscopic repair of perforated peptic ulcer: simple closure versus omentopexy. J Surg Res. 2017;220:341–345. doi: 10.1016/j.jss.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Bertleff M.J., Lange J.F. Laparoscopic correction of perforated peptic ulcer: first choice? A review of literature. Surg Endosc. 2010;24:1231–1239. doi: 10.1007/s00464-009-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed O., Walters N.J., Day R.M., Kim H.W., Knowles J.C. Evaluation of decellularization protocols of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014;10:5043–5054. doi: 10.1016/j.actbio.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 34.Kitahara H., Yagi H., Tajima K., Okamoto K., Yoshitake A., Aeba R. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact Cardiovasc Thorac Surg. 2016;22:571–579. doi: 10.1093/icvts/ivw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Meier E.M., Tian S., Lei I., Liu L., Xian S. Transplantation of lsl1+ cardiac progenitor cells in small intestinal submucosa improves infarcted heart function. Stem Cell Res Ther. 2017;8:230. doi: 10.1186/s13287-017-0675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF, Prockop DJ. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type 1 procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983;22:5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- 40.Niu D., Wei H.J., Lin L., George H., Wang T., Lee I.H. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–1307. doi: 10.1126/science.aan4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekine H., Shimizu T., Hobo K., Sekiya S., Yang J., Yamato M. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 42.Sasagawa T., Shimizu T., Sekiya S., Haraguchi Y., Yamato M., Sawa Y. Design of prevascularized three-dimensional cell-dense tissue using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1