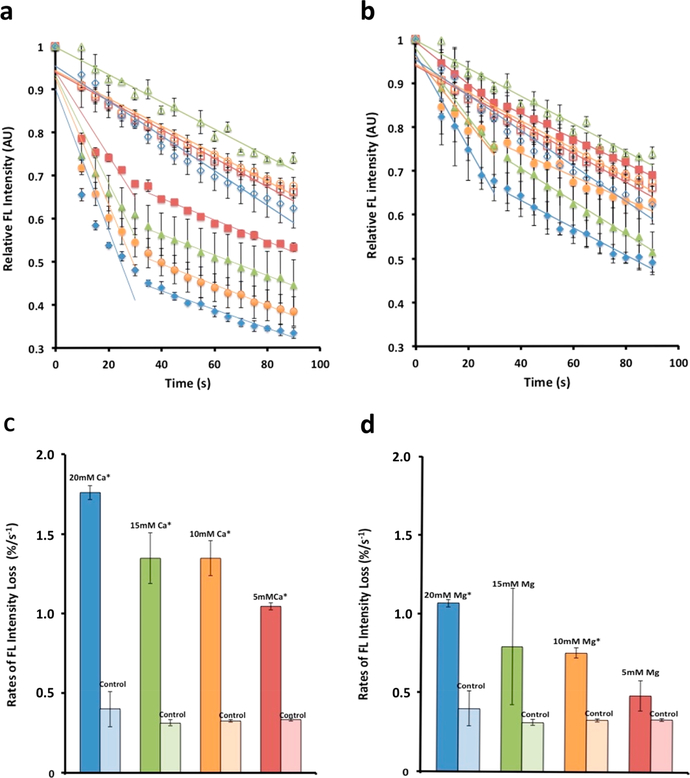
Figure 1.
Cadmium telluride quantum dots adhered to myosin molecules function as nanoscale thermometers, enabling the detection of heat loss from the motor protein on binding to calcium or magnesium. Binding of calcium to bovine cardiac (BC) myosin lowers the enthalpy of the molecule to a greater degree than magnesium. (a–d) Binding of calcium to bovine cardiac (BC) myosin lowers the energy state of the motor protein greater than magnesium. (a) Cadmium telluride quantum dots associated with the myosin molecule and used as molecular thermometers are able to detect mK changes in temperature of the myosin molecule. Changes in temperature are reflected as a change in fluorescence. The greater the heat loss, the lower the fluorescence.11 Calcium concentrations used were 5 mM (solid red squares), 10 mM (solid green triangles), 15 mM (solid orange circles), and 20 mM (solid blue diamonds). Color coded corresponding controls were empty symbols. This calcium and magnesium dependent drop in fluorescence is a measure of heat release. Real-time fluorimetry demonstrates the extent and rate of this heat loss. (b) There is similarly a magnesium dependent drop in fluorescence, however, to a lesser extent than calcium. (c, d) Rate of fluorescence loss in the first 30 s following addition of calcium or magnesium. (c) Note that the rate of heat release in the presence of increasing calcium concentration is 30% greater than is observed with (d) magnesium. (Data presented is MEAN ± SEM, n = 3, *p < 0.005). Similar study using rabbit skeletal (RS) myosin is presented in Figure 1S (Supporting Information).