Abstract
Background
This study aimed to explore the correlation of baseline, procedural, and postprocedure characteristics with the risk of rapid angiographic stenotic progression (RASP) and restenosis in coronary artery disease (CAD) patients after percutaneous coronary intervention (PCI) with drug‐eluting stents implantation.
Methods
Two hundred fourteen CAD patients underwent PCI with drug‐eluting stents implantation were consecutively enrolled. Baseline, procedural, and postprocedure characteristics of patients were collected for analysis. Coronary angiography was performed to evaluate coronary stenosis before PCI and at 12 months after PCI. RASP of nontarget lesions and restenosis of stent‐implanted target lesions were then assessed.
Results
37.8% CAD patients occurred RASP at 12 months after PCI and compared to non‐RASP group, RASP group presented with increased diabetes mellitus (DM) complication, higher concentration of serum uric acid (SUA), cardiac troponin I, N‐terminal probrain natriuretic peptide, and high sensitive C‐reactive protein (hs‐CRP) as well as elevated occurrence of multivessel artery lesions. In addition, DM, SUA, hs‐CRP, and multivessel artery lesions independently predicted high RASP risk. For restenosis, 21.0% patients occurred restenosis at 12 months after PCI, and patients in restenosis group presented with increased hypertension and DM occurrence, higher concentrations of SUA, LDL‐C, and hs‐CRP, as well as longer target lesion and length of stent in surgery compared to nonrestenosis group. Also, DM, SUA, LDL‐C, hs‐CRP, and length of target lesion independently predicted increased restenosis risk.
Conclusion
Diabetes mellitus, SUA, and hs‐CRP are potential predictive factors for increased risk of both RASP and restenosis in CAD patients underwent PCI and drug‐eluting stents implantation.
Keywords: coronary artery disease, drug‐eluting stents, hs‐CRP, RASP, restenosis
1. INTRODUCTION
Coronary artery disease (CAD), mainly caused by atherosclerosis and plaque formation in coronary arteries, is the most common type of ischemic cardiovascular disorder, which has become the leading cause of global death along with its consequent complications including angina, heart failure, and myocardial infarction.1 Drug‐eluting stents in CAD treatment are scaffolds placed in coronary artery that locally elute anti‐proliferative and anti‐inflammatory drugs to counteract vessel wall recoil and reduce restenosis after widening the coronary arteries with percutaneous coronary intervention (PCI).2, 3, 4, 5 Although PCI with drug‐eluting stents implementation has improved clinical outcomes in CAD patients, the occurrence rate of restenosis remains high in CAD patients treated with drug‐eluting stents, and complications particularly rapid angiographic stenotic progression (RASP) of the nontarget lesion are frequently reported to accompany PCI.6 Therefore, it is of great need to explore potential markers for prediction of RASP and restenosis risks to improve quality of life for CAD patients underwent PCI with drug‐eluting stents implementation.
Various clinical indexes including precipitating factors (eg, smoking and inflammation), biochemical parameters (eg, lipoprotein and cholesterol), coronary angiograms (eg, lesion size and number), or diseases history (eg, obesity, diabetes mellitus (DM), and hypertension) have been widely used for forecasting disease risk, monitoring disease severity, and predicting prognosis in CAD patients.3, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, limited information is available about the function of these clinical indexes on the risk of RASP and restenosis in CAD patients after PCI with drug‐eluting stents implantation. In the present study, we collected comprehensive baseline, procedural, and postprocedure characteristics of patients and aimed to explore their correlation with the risk of RASP at the nontarget lesion and restenosis of target lesion in CAD patients after PCI with drug‐eluting stents implantation.
2. MATERIALS AND METHODS
2.1. Patients
Two hundred fourteen CAD patients who underwent PCI with drug‐eluting stents implantation at Department of Cardiology, The Second Hospital of Hebei Medical University between November 2014 and October 2016 were consecutively enrolled in this prospective cohort study. The inclusion criteria were as follows: (a) Diagnosed as severe CAD according to the coronary angiography, which was defined as the presence of ≥70% of luminal stenosis in at least one major coronary vessel; (b) Age above 18 years old; (c) About to undergo PCI with drug‐eluting stents implantation (depending on FFR value (FFR <0.75), patients’ personal willingness and financial capability); (d) Able to be followed up regularly. The exclusion criteria included: (a) Occurrence of acute myocardial infarction or unstable angina pectoris within 6 months; (b) History of PCI or coronary artery bypass grafting; (c) History of major surgery; (d) Fatal arrhythmias or uncontrolled hypertension (systolic pressure ≥160 mm Hg and/or diastolic pressure ≥105 mm Hg); (e) Liver or kidney dysfunction; (f) Complicated with diseases or conditions leading to changes in inflammatory markers such as malignant tumors, inflammatory diseases, autoimmune diseases, untreated thyroid diseases, estrogen replacement therapy, fever (>37.5°C), surgery within 3 months, trauma, or infectious diseases, thromboembolic diseases, valvular diseases, cardiomyopathy, heart transplant, congestive heart failure (New York Heart Association functional class III‐IV), or left ventricular dysfunction (left ventricular ejection fraction (LVEF) <40%); (g) Previously took anti‐inflammatory drugs or immunosuppressive drugs within 3 months; (h) Contraindications to aspirin or clopidogrel; (i) Pregnant women or lactating women.
2.2. Ethics statement
This study was approved by the Institutional Review Board of The Second Hospital of Hebei Medical University and performed in accordance with the Declaration of Helsinki. Written informed consents were obtained from all patients before enrollment.
2.3. Data collection
Baseline, procedural, and postprocedure characteristics of patients were collected including: (a) Demographic information: age, gender, and body mass index (BMI); (b) Cardiovascular risk factors: smoke, hypertension, DM, hypercholesteremia, hyperuricemia, and family history of CAD; (c) LVEF; (d) Laboratory tests: mean arterial pressure (MAP), fasting blood‐glucose (FBG), serum creatinine (Scr), serum uric acid (SUA), cardiac troponin I (cTnI), N‐terminal probrain natriuretic peptide (NT‐proBNP), triglyceride (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), high‐sensitivity C‐reactive protein (hs‐CRP), erythrocyte sedimentation rate (ESR), white blood cell (WBC), and neutrophil; (e) Coronary angiography findings: multivessel artery lesions, target lesion at left anterior descending branch (LAD), target lesion at left circumflex artery (LCX), target lesion at right coronary artery (RCA), percentage of patients with two target lesions, stenosis degree of target lesion, and length of target lesion; (f) PCI surgery parameters: bifurcation requiring double wiring, length of stent, diameter of stent, time of stent dilation, and balloon dilation pre‐stent; (g) Drugs used after surgery: aspirin, nitrates, statins, β‐receptor blockers, angiotensin converting enzymes inhibitors/angiotensin receptor blockers (ACEIs/ARBs), and calcium channel blockers.
2.4. Coronary angiography
Coronary angiography was performed to evaluate coronary stenosis before PCI, immediately after PCI and at 12 months after PCI. The operation and projection position of two coronary angiographies were consistent to obtain comparable diagnostic angiograms. Coronary angiogram was obtained with the use of Angiography System (Siemens, Germany), and quantitative coronary angiographic analysis of atherosclerotic plaques and luminal stenosis was performed using supporting software system according to the standard operating procedure. Before angiography, 0.5 mg glycerin trinitrate was given to the patient, and 5 minutes later the contrast agent was injected, then coronary angiogram was acquired. Coronary angiogram was analyzed and evaluated by two independent experienced specialists.
2.5. PCI and drug‐eluting stents implantation
In the present study, PCI, drug‐eluting stents implantation, and perioperative management were carried out according to the recommendations of the Guideline of PCI. Aspirin (≥75 mg/d) and clopidogrel (300 mg at first time, then 75 mg/d for at least 9 months) were administrated before and after PCI, meanwhile, heparin was given at a dose of 10 000 UI by intravenous injection to keep activated clotting time before PCI. If necessary, balloon dilation was conducted prior to stent implantation. All patients received rapamycin‐eluting stent or paclitaxel‐eluting stent implantation according to the disease conditions and personal willingness. After PCI and drug‐eluting stents implantation, patients received standard medication recommended by the guide including aspirin, nitrates, statins, β‐receptor blockers, ACEIs/ARBs, calcium channel blockers, and so on.
2.6. Assessments
Rapid angiographic stenotic progression (RASP) of nontarget lesions and restenosis of stent‐implanted target lesions was evaluated at 12 months post‐PCI. RASP of nontarget lesion was defined as the occurrence of any of the following conditions: (a) The increase of stenosis ≥10% at 12 months if the original stenosis was ≥50% before PCI; (b) The increase of stenosis ≥30% at 12 months if the original stenosis was <50% before PCI; (c) Newly developed stenosis ≥30% at 12 months if no original stenosis existed before PCI; (d) The stenosis aggravated and turned to complete occlusion lesion at 12 months. Restenosis of target lesion was defined as: at 12 months after PCI, lumen stenosis of the stent‐implanted site exceeded 50% compared with lumen assessed immediately after PCI.
2.7. Statistics analysis
SPSS 21.0 statistical software (SPSS Inc, Chicago, IL, USA) was used for statistical data processing. Count data were expressed as count (percentage); Measurement data were presented as mean ± standard deviation (normally distributed data) or median (25th‐75th quantiles) (skewed distributed data). Comparison between two groups was determined by chi‐square test, t test, or Wilcoxon rank sum test. Factors affecting RASP or restenosis occurrence were determined by univariate and multivariate logistic regression model analysis, and the Forward Stepwise (Conditional) method was used in the multivariate logistic regression model. P value <0.05 was considered significant.
3. RESULTS
3.1. Study flow
Initially, a total of 457 CAD patients were invited, whereas 81 patients were excluded including 64 patients missed the invitation and 17 patients declined to attend prescreening procedure. 376 patients were then screened for eligibility, while 90 patients were excluded (including 71 patients did not meet inclusion criteria and 19 patients disagreed to sign informed consents). The remaining 286 CAD patients were recruited and successfully underwent PCI with drug‐eluting stents implantation. During the 12 months follow‐up period after PCI, 72 patients lost follow‐up, leaving 214 patients for angiographic follow‐up at 12 months after PCI. Finally, there were 81 CAD patients presented with RASP and 45 CAD patients presented with restenosis (Figure 1).
Figure 1.
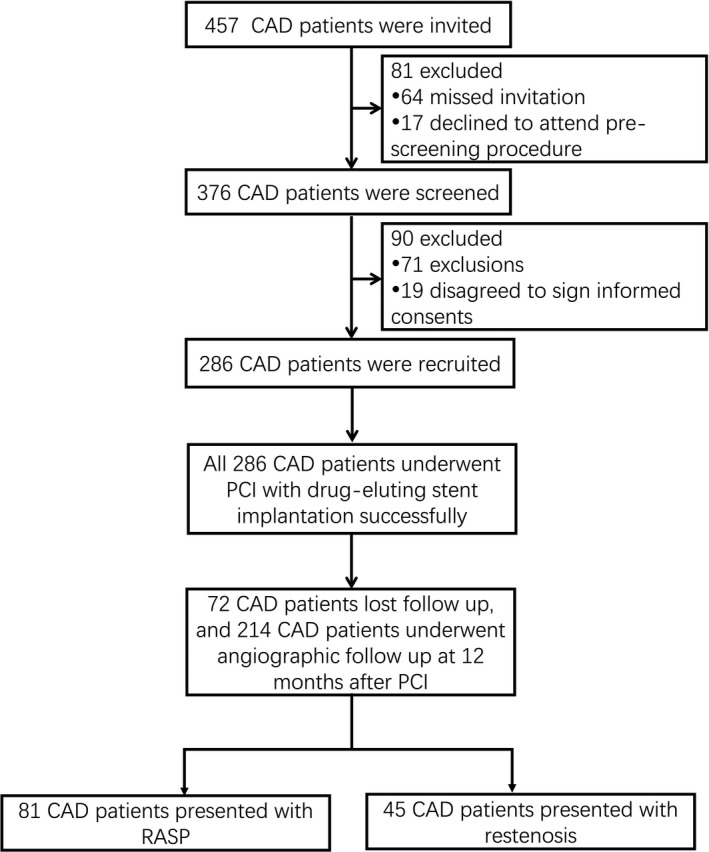
Study flow
3.2. Patients’ baseline, operational, and postprocedure characteristics
The mean age of 214 CAD patients (including 171 males and 43 females) was 58.7 ± 8.8 years (Table 1). The numbers of patients with hypertension, DM, hypercholesteremia, hyperuricemia, and family history of CAD were 161 (75.2%), 59 (27.6%), 127 (59.3%), 91 (42.5%), and 39 (18.2%), respectively. And the mean LVEF was 64.98 ± 6.52%. The mean SUA (337.50 ± 82.64 μmol/L), median cTnI (0.029 (0.017‐0.041) ng/mL), median NT‐proBNP (0.076 (0.040‐0.116) ng/mL), mean LDL‐C (2.80 ± 0.64 mmol/L), and median hs‐CRP (2.24 (0.99‐4.14) mg/L) were recorded from laboratory test. During surgery, 73 (34.1%) patients had bifurcation requiring double wiring, the median length of stent, diameter of stent, and time of stent dilation were 38.00 (31.00‐44.00) mm, 3.20 (3.00‐3.40) mm, and 15.00 (13.00‐18.00) seconds, respectively, and the number of patients received balloon dilation pre‐stent was 69 (32.2%). After surgery, numbers of patients received aspirin, nitrates, statins, β‐receptor blockers, ACEIs/ARBs, and calcium channel blockers were 214 (100.0%), 205 (95.8%), 208 (97.2%), 196 (91.6%), 150 (70.1%), and 75 (35.0%), respectively. Other baseline characteristics were listed in Table 1.
Table 1.
Baseline, operational, and postprocedure characteristics of CAD patients
| Characteristics | CAD patients (N = 214) |
|---|---|
| Age (y) | 58.7 ± 8.8 |
| Gender (male/female) | 171/43 |
| BMI (kg/m2) | 25.9 ± 3.1 |
| Smoke (n/%) | 76 (35.5) |
| Hypertension (n/%) | 161 (75.2) |
| DM (n/%) | 59 (27.6) |
| Hypercholesteremia (n/%) | 127 (59.3) |
| Hyperuricemia (n/%) | 91 (42.5) |
| Family history of CAD (n/%) | 39 (18.2) |
| LVEF (%) | 64.98 ± 6.52 |
| Laboratory tests | |
| MAP (mm Hg) | 104.26 ± 17.50 |
| FBG (mmol/L) | 5.73 (5.14‐6.56) |
| Glycated hemoglobin (%) | 6.25 ± 1.86 |
| Scr (μmol/L) | 80.78 ± 16.71 |
| SUA (μmol/L) | 337.50 ± 82.64 |
| cTnI (ng/mL) | 0.029 (0.017‐0.041) |
| NT‐proBNP (ng/mL) | 0.076 (0.040‐0.116) |
| TG (mmol/L) | 1.73 (1.00‐2.46) |
| TC (mmol/L) | 4.65 ± 1.00 |
| LDL‐C (mmol/L) | 2.80 ± 0.64 |
| HDL‐C (mmol/L) | 1.03 ± 0.27 |
| Hs‐CRP (mg/L) | 2.24 (0.99‐4.14) |
| ESR (mm/L) | 6.94 ± 3.92 |
| WBC (109/L) | 6.04 ± 1.47 |
| Neutrophil (109/L) | 3.47 ± 1.01 |
| Coronary angiography findings | |
| Multivessel artery lesions (n/%) | 160 (74.8) |
| Target lesion at LAD (n/%) | 120 (56.1) |
| Target lesion at LCX (n/%) | 80 (37.4) |
| Target lesion at RCA (n/%) | 75 (35.0) |
| Patients with two target lesions (n/%) | 61 (28.5) |
| Stenosis degree of target lesion (%) | 88.00 (84.75‐92.00) |
| Length of target lesion (mm) | 34.00 (27.00‐40.25) |
| PCI surgery parameters | |
| Bifurcation requiring double wiring (n/%) | 73 (34.1) |
| Length of stent (mm) | 38.00 (31.00‐44.00) |
| Diameter of stent (mm) | 3.20 (3.00‐3.40) |
| Time of stent dilation (s) | 15.00 (13.00‐18.00) |
| Balloon dilation pre‐stent (n/%) | 69 (32.2) |
| Drugs used after surgery | |
| Aspirin (n/%) | 214 (100.0) |
| Nitrates (n/%) | 205 (95.8) |
| Statins (n/%) | 208 (97.2) |
| β‐receptor blockers (n/%) | 196 (91.6) |
| ACEIs/ARBs (n/%) | 150 (70.1) |
| Calcium channel blockers (n/%) | 75 (35.0) |
CAD, coronary artery disease; BMI, body mass index; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; FBG, fasting blood‐glucose; Scr, serum creatinine; SUA, serum uric acid; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; TG, triglyceride; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; LAD, left anterior descending branch; LCX, left circumflex artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; ACEIs/ARBs, angiotensin converting enzymes inhibitors/angiotensin receptor blockers.
Data were presented as mean value ± standard deviation, median (25th‐75thquantiles), or count (percentage).
3.3. Comparison of characteristics between RASP group and non‐RASP group
A total of 81 (37.8%) CAD patients occurred RASP at 12 months after PCI, according to which patients were divided into RASP (N = 81) and non‐RASP groups (N = 133), and we found that RASP group presented with increased DM complication (P = 0.035), higher concentrations of SUA (P = 0.006), cTnI (P = 0.022), NT‐proBNP (P = 0.026), hs‐CRP (P = 0.001), and raised occurrence of multivessel artery lesions (P = 0.001) as well as bifurcation requiring double wiring (P = 0.001) compared to non‐RASP group, while no difference in other characteristics between the two groups was observed (Table 2).
Table 2.
Comparison of characteristics between RASP patients and non‐RASP patients
| Characteristics | RASP patients (N = 81) | Non‐RASP patients (N = 133) | P value |
|---|---|---|---|
| Age (y) | 60.1 ± 8.7 | 58.2 ± 8.8 | 0.129 |
| Gender (male/female) | 61/20 | 110/23 | 0.190 |
| BMI (kg/m2) | 26.3 ± 3.1 | 25.6 ± 3.0 | 0.107 |
| Smoke (n/%) | 30 (37.0) | 46 (34.6) | 0.716 |
| Hypertension (n/%) | 64 (79.0) | 97 (72.9) | 0.318 |
| DM (n/%) | 29 (35.8) | 30 (22.6) | 0.035 |
| Hypercholesteremia (n/%) | 54 (66.7) | 73 (54.9) | 0.089 |
| Hyperuricemia (n/%) | 33 (40.7) | 58 (43.6) | 0.681 |
| Family history of CAD (n/%) | 16 (19.8) | 23 (17.3) | 0.651 |
| LVEF (%) | 65.06 ± 5.8 | 64.93 ± 7.0 | 0.876 |
| Laboratory tests | |||
| MAP (mm Hg) | 103.68 ± 17.45 | 104.61 ± 17.58 | 0.707 |
| FBG (mmol/L) | 5.75 (5.28‐6.60) | 5.70 (5.02‐6.52) | 0.534 |
| Glycated hemoglobin (%) | 6.34 ± 1.75 | 6.20 ± 1.93 | 0.575 |
| Scr (μmol/L) | 79.17 ± 16.94 | 81.76 ± 16.55 | 0.272 |
| SUA (μmol/L) | 357.10 ± 73.46 | 325.56 ± 85.56 | 0.006 |
| cTnI (ng/mL) | 0.033 (0.024‐0.043) | 0.027 (0.014‐0.039) | 0.022 |
| NT‐proBNP (ng/mL) | 0.088 (0.053‐0.120) | 0.066 (0.036‐0.108) | 0.026 |
| TG (mmol/L) | 1.83 (1.07‐2.53) | 1.68 (0.99‐2.41) | 0.244 |
| TC (mmol/L) | 4.55 ± 1.03 | 4.71 ± 0.97 | 0.240 |
| LDL‐C (mmol/L) | 2.79 ± 0.60 | 2.80 ± 0.61 | 0.901 |
| HDL‐C (mmol/L) | 0.99 ± 0.28 | 1.05 ± 0.26 | 0.100 |
| Hs‐CRP (mg/L) | 3.12 (1.05‐6.10) | 1.88 (0.93‐3.19) | 0.001 |
| ESR (mm/L) | 7.35 ± 4.23 | 6.69 ± 3.71 | 0.235 |
| WBC (109/L) | 6.13 ± 1.39 | 5.98 ± 1.52 | 0.486 |
| Neutrophil (109/L) | 3.62 ± 1.02 | 3.38 ± 0.99 | 0.098 |
| Coronary angiography findings | |||
| Multivessel artery lesions (n/%) | 71 (87.7) | 89 (66.9) | 0.001 |
| Target lesion at LAD (n/%) | 42 (51.9) | 78 (58.6) | 0.331 |
| Target lesion at LCX (n/%) | 25 (30.9) | 55 (41.4) | 0.124 |
| Target lesion at RCA (n/%) | 33 (40.7) | 42 (31.6) | 0.173 |
| Patients with two target lesions (n/%) | 19 (23.5) | 42 (31.6) | 0.202 |
| Stenosis degree of target lesion (%) | 87.00 (84.00‐92.00) | 88.00 (85.00‐92.00) | 0.643 |
| Length of target lesion (mm) | 36.00 (28.50‐43.00) | 34.00 (27.00‐39.50) | 0.113 |
| PCI surgery parameters | |||
| Bifurcation requiring double wiring (n/%) | 39 (48.1) | 34 (25.6) | 0.001 |
| Length of stent (mm) | 39.00 (32.00‐47.00) | 37.00 (31.00‐43.00) | 0.123 |
| Diameter of stent (mm) | 3.30 (3.00‐3.40) | 3.20 (3.00‐3.40) | 0.223 |
| Time of stent dilation (s) | 15.00 (12.00‐18.00) | 16.00 (13.00‐18.00) | 0.286 |
| Balloon dilation pre‐stent (n/%) | 26 (32.1) | 43 (32.3) | 0.972 |
| Drugs used after surgery | |||
| Aspirin (n/%) | 81 (100.0) | 133 (100.00) | ‐ |
| Nitrates (n/%) | 78 (96.3) | 127 (95.5) | 0.775 |
| Statins (n/%) | 79 (97.5) | 129 (97.0) | 0.817 |
| β‐receptor blockers (n/%) | 73 (90.1) | 123 (92.5) | 0.547 |
| ACEIs/ARBs (n/%) | 57 (70.4) | 93 (69.9) | 0.945 |
| Calcium channel blockers (n/%) | 27 (33.3) | 48 (36.1) | 0.682 |
RASP, rapid angiographic stenotic progression; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; FBG, fasting blood‐glucose; Scr, serum creatinine; SUA, serum uric acid; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; TG, triglyceride; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; LAD, left anterior descending branch; LCX, left circumflex artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; ACEIs/ARBs, angiotensin converting enzymes inhibitors/angiotensin receptor blockers.
Data were presented as mean value ± standard deviation, median (25th‐75th quantiles), or count (percentage). Comparison between two groups was determined by t test, Wilcoxon rank sum test, or chi‐square test. P value <0.05 was considered significant. Statistically significant values were shown in bold font.
3.4. Logistic regression analysis for factors affecting RASP occurrence in CAD patients
Univariate regression model analysis revealed that DM (P = 0.037), SUA (P = 0.008), hs‐CRP (P < 0.001), multivessel artery lesions (P = 0.001), and bifurcation requiring double wiring (P = 0.001) were positively correlated with the risk of RASP (Table 3). And multivariate logistic regression model analysis with the Forward Stepwise (Conditional) method further illustrated that DM (P = 0.022), SUA (P = 0.011), hs‐CRP (P < 0.001), multivessel artery lesions (P = 0.010), and bifurcation requiring double wiring (P = 0.001) independently predicted higher risk of RASP (Table 4).
Table 3.
Factors affecting RASP occurrence by univariate logistic regression model analysis
| Parameters | Univariate logistic regression | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| Age | 0.130 | 1.025 | 0.993 | 1.058 |
| Gender (male) | 0.192 | 0.638 | 0.324 | 1.254 |
| BMI | 0.108 | 1.077 | 0.984 | 1.179 |
| Smoke | 0.716 | 1.113 | 0.626 | 1.978 |
| Hypertension | 0.319 | 1.397 | 0.724 | 2.697 |
| DM | 0.037 | 1.915 | 1.041 | 3.523 |
| Hypercholesteremia | 0.090 | 1.644 | 0.925 | 2.920 |
| Hyperuricemia | 0.681 | 0.889 | 0.508 | 1.557 |
| Family history of CAD | 0.651 | 1.177 | 0.580 | 2.390 |
| LVEF | 0.881 | 1.003 | 0.962 | 1.047 |
| Laboratory tests | ||||
| MAP | 0.706 | 0.997 | 0.981 | 1.013 |
| FBG | 0.713 | 1.043 | 0.833 | 1.306 |
| Glycated hemoglobin | 0.574 | 1.043 | 0.900 | 1.210 |
| Scr | 0.271 | 0.991 | 0.974 | 1.007 |
| SUA | 0.008 | 1.005 | 1.001 | 1.008 |
| cTnI | 0.119 | ‐ | 0.053 | ‐ |
| NT‐proBNP | 0.208 | 19.440 | 0.191 | 1976 |
| TG | 0.253 | 1.198 | 0.879 | 1.632 |
| TC | 0.240 | 0.845 | 0.637 | 1.119 |
| LDL‐C | 0.900 | 0.973 | 0.631 | 1.499 |
| HDL‐C | 0.101 | 0.416 | 0.146 | 1.187 |
| Hs‐CRP | <0.001 | 1.390 | 1.207 | 1.601 |
| ESR | 0.235 | 1.044 | 0.973 | 1.121 |
| WBC | 0.484 | 1.069 | 0.886 | 1.291 |
| Neutrophil | 0.099 | 1.265 | 0.957 | 1.671 |
| Coronary angiography findings | ||||
| Multivessel artery lesions | 0.001 | 3.510 | 1.651 | 7.462 |
| Target lesion at LAD | 0.332 | 0.759 | 0.436 | 1.324 |
| Target lesion at LCX | 0.125 | 0.633 | 0.353 | 1.136 |
| Target lesion at RCA | 0.174 | 1.490 | 0.839 | 2.646 |
| Patients with two target lesions | 0.203 | 0.664 | 0.353 | 1.248 |
| Stenosis degree of target lesion | 0.924 | 0.998 | 0.952 | 1.046 |
| Length of target lesion | 0.101 | 1.027 | 0.995 | 1.061 |
| PCI surgery parameters | ||||
| Bifurcation requiring double wiring | 0.001 | 2.704 | 1.507 | 4.851 |
| Length of stent | 0.111 | 1.027 | 0.994 | 1.060 |
| Diameter of stent | 0.313 | 1.587 | 0.646 | 3.896 |
| Time of stent dilation | 0.289 | 0.963 | 0.898 | 1.033 |
| Balloon dilation pre‐stent | 0.972 | 0.989 | 0.548 | 1.787 |
| Drugs used after surgery | ||||
| Aspirin | ‐ | ‐ | ‐ | ‐ |
| Nitrates | 0.776 | 1.228 | 0.299 | 5.053 |
| Statins | 0.817 | 1.225 | 0.219 | 6.842 |
| β‐receptor blockers | 0.548 | 0.742 | 0.280 | 1.964 |
| ACEIs/ARBs | 0.945 | 1.022 | 0.558 | 1.869 |
| Calcium channel blockers | 0.682 | 0.885 | 0.495 | 1.584 |
RASP, rapid angiographic stenotic progression; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; FBG, fasting blood‐glucose; Scr, serum creatinine; SUA, serum uric acid; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; TG, triglyceride; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; LAD, left anterior descending branch; LCX, left circumflex artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; ACEIs/ARBs, angiotensin converting enzymes inhibitors/angiotensin receptor blockers.
Data were presented as P value, OR (odds ratio), and 95% CI (confidence interval). P value <0.05 was considered significant. Factors affecting RASP occurrence were determined by univariate logistic regression model analysis, and “‐” indicated that there was no statistical performance due to lack of events. Statistically significant values were shown in bold font.
Table 4.
Factors affecting RASP occurrence by multivariate logistic regression model analysis with the Forward Stepwise (Conditional) method
| Parameters | Multivariate logistic regression | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| DM | 0.022 | 2.322 | 1.131 | 4.765 |
| SUA | 0.011 | 1.005 | 1.001 | 1.009 |
| Hs‐CRP | <0.001 | 1.393 | 1.193 | 1.627 |
| Multivessel artery lesions | 0.010 | 2.991 | 1.305 | 6.858 |
| Bifurcation requiring double wiring | 0.001 | 3.082 | 1.564 | 6.076 |
RASP, rapid angiographic stenotic progression; DM, diabetes mellitus; SUA, serum uric acid; Hs‐CRP, high‐sensitivity C‐reactive protein.
Data were presented as P value, OR (odds ratio), and 95% CI (confidence interval). P value <0.05 was considered significant. Factors affecting RASP occurrence were determined by multivariate logistic regression model analysis with the Forward Stepwise (Conditional) method. Statistically significant values were shown in bold font.
3.5. Comparison of characteristics between restenosis group and nonrestenosis group
Totally 45 (21.0%) patients occurred restenosis at 12 months after PCI, depending on which patients were divided into restenosis group (N = 45) and nonrestenosis group (N = 169), and in restenosis group, patients presented with increased hypertension (P = 0.017) and DM (P = 0.013) occurrence, higher concentrations of SUA (P = 0.020), LDL‐C (P = 0.010) and hs‐CRP (P < 0.001), longer length of target lesion (P = 0.029) and length of stent (P = 0.026) used in PCI surgery as well as higher rate of bifurcation requiring double wiring (P = 0.002), whereas no difference was found in other characteristics between the two groups (Table 5).
Table 5.
Comparison of characteristics between restenosis patients and nonrestenosis patients
| Characteristics | Restenosis patients (N = 45) | Nonrestenosis patients (N = 169) | P value |
|---|---|---|---|
| Age (y) | 59.5 ± 9.1 | 58.7 ± 8.7 | 0.591 |
| Gender (male/female) | 36/9 | 135/34 | 0.986 |
| BMI (kg/m2) | 26.0 ± 2.9 | 25.8 ± 3.1 | 0.690 |
| Smoke (n/%) | 13 (28.9) | 63 (37.3) | 0.296 |
| Hypertension (n/%) | 40 (88.9) | 121 (71.6) | 0.017 |
| DM (n/%) | 19 (42.2) | 40 (23.7) | 0.013 |
| Hypercholesteremia (n/%) | 27 (60.0) | 100 (59.2) | 0.920 |
| Hyperuricemia (n/%) | 17 (37.8) | 74 (43.8) | 0.469 |
| Family history of CAD (n/%) | 7 (15.6) | 32 (18.9) | 0.602 |
| LVEF (%) | 64.33 ± 6.82 | 65.15 ± 6.45 | 0.458 |
| Laboratory tests | |||
| MAP (mm Hg) | 101.44 ± 17.14 | 105.01 ± 17.57 | 0.226 |
| FBG (mmol/L) | 5.68 (5.28‐6.36) | 5.76 (5.04‐6.57) | 0.864 |
| Glycated hemoglobin (%) | 6.35 ± 1.87 | 6.23 ± 1.87 | 0.691 |
| Scr (μmol/L) | 79.17 ± 19.00 | 81.47 ± 16.04 | 0.240 |
| SUA (μmol/L) | 362.97 ± 91.88 | 330.72 ± 78.92 | 0.020 |
| cTnI (ng/mL) | 0.029 (0.016‐0.042) | 0.029 (0.017‐0.041) | 0.973 |
| NT‐proBNP (ng/mL) | 0.077 (0.037‐0.125) | 0.074 (0.021‐0.111) | 0.825 |
| TG (mmol/L) | 1.88 (1.13‐2.58) | 1.72 (0.95‐2.43) | 0.233 |
| TC (mmol/L) | 4.85 ± 1.05 | 4.60 ± 0.98 | 0.133 |
| LDL‐C (mmol/L) | 3.02 ± 0.71 | 2.74 ± 0.61 | 0.010 |
| HDL‐C (mmol/L) | 1.08 ± 0.30 | 1.02 ± 0.26 | 0.179 |
| Hs‐CRP (mg/L) | 5.18 (3.66‐6.79) | 1.67 (0.77‐3.04) | <0.001 |
| ESR (mm/L) | 6.00 ± 3.96 | 7.19 ± 3.88 | 0.069 |
| WBC (109/L) | 5.84 ± 1.30 | 6.09 ± 1.51 | 0.317 |
| Neutrophil (109/L) | 3.49 ± 1.17 | 3.46 ± 0.97 | 0.855 |
| Coronary angiography findings | |||
| Multivessel artery lesions (n/%) | 37 (82.2) | 123 (72.8) | 0.195 |
| Target lesion at LAD (n/%) | 27 (60.0) | 93 (55.0) | 0.550 |
| Target lesion at LCX (n/%) | 15 (33.3) | 65 (38.5) | 0.527 |
| Target lesion at RCA (n/%) | 16 (35.6) | 59 (34.9) | 0.936 |
| Patients with two target lesions (n/%) | 13 (28.9) | 48 (28.4) | 0.949 |
| Stenosis degree of target lesion (%) | 88.00 (85.00‐92.00) | 88.00 (84.00‐92.00) | 0.669 |
| Length of target lesion (mm) | 37.00 (30.50‐34.50) | 33.00 (27.00‐40.00) | 0.029 |
| PCI surgery parameters | |||
| Bifurcation requiring double wiring (n/%) | 24 (53.3) | 49 (29.0) | 0.002 |
| Length of stent (mm) | 41.00 (33.50‐47.00) | 37.00 (31.00‐43.00) | 0.026 |
| Diameter of stent (mm) | 3.20 (3.10‐3.45) | 3.20 (3.00‐3.40) | 0.377 |
| Time of stent dilation (s) | 15.00 (12.00‐18.00) | 15.00 (13.00‐18.00) | 0.322 |
| Balloon dilation pre‐stent (n/%) | 16 (35.6) | 53 (31.4) | 0.593 |
| Drugs used after surgery | |||
| Aspirin (n/%) | 45 (100.0) | 169 (100.00) | ‐ |
| Nitrates (n/%) | 42 (93.3) | 163 (96.4) | 0.355 |
| Statins (n/%) | 43 (95.6) | 165 (97.6) | 0.453 |
| β‐receptor blockers (n/%) | 40 (88.9) | 156 (92.3) | 0.463 |
| ACEIs/ARBs (n/%) | 32 (71.1) | 118 (69.8) | 0.867 |
| Calcium channel blockers (n/%) | 17 (37.8) | 58 (34.3) | 0.666 |
BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; FBG, fasting blood‐glucose; Scr, serum creatinine; SUA, serum uric acid; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; TG, triglyceride; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; LAD, left anterior descending branch; LCX, left circumflex artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; ACEIs/ARBs, angiotensin converting enzymes inhibitors/angiotensin receptor blockers.
Data were presented as mean value ± standard deviation, median (25th‐75th quantiles), or count (percentage). Comparison between two groups was determined by t test, Wilcoxon rank sum test or chi‐square test. P value <0.05 was considered significant. Statistically significant values were shown in bold font.
3.6. Logistic regression analysis for factors affecting restenosis occurrence in CAD patients
Univariate regression model analysis revealed that hypertension (P = 0.022), DM (P = 0.015), SUA level (P = 0.021), LDL‐C level (P = 0.011), hs‐CRP level (P =< 0.001), length of target lesion (P = 0.032), length of stent used in PCI surgery (P = 0.031), and bifurcation requiring double wiring (P = 0.003) were risk factors for restenosis occurrence (Table 6). In addition, multivariate logistic regression analysis with the Forward Stepwise (conditional) method exhibited that DM (P < 0.001), SUA (P = 0.049), LDL‐C (P < 0.001), hs‐CRP (P < 0.001), length of target lesion (P = 0.002), and bifurcation requiring double wiring (P = 0.009) were independent factors for predicting increased occurrence of restenosis (Table 7).
Table 6.
Factors affecting restenosis occurrence by univariate logistic regression model analysis
| Parameters | Univariate logistic regression | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| Age | 0.589 | 1.010 | 0.973 | 1.049 |
| Gender (male) | 0.986 | 1.007 | 0.443 | 2.291 |
| BMI | 0.689 | 1.022 | 0.919 | 1.136 |
| Smoke | 0.298 | 0.684 | 0.334 | 1.399 |
| Hypertension | 0.022 | 3.174 | 1.182 | 8.524 |
| DM | 0.015 | 2.357 | 1.182 | 4.697 |
| Hypercholesteremia | 0.920 | 1.035 | 0.529 | 2.024 |
| Hyperuricemia | 0.469 | 0.779 | 0.397 | 1.531 |
| Family history of CAD | 0.602 | 0.789 | 0.323 | 1.927 |
| LVEF | 0.456 | 0.981 | 0.932 | 1.032 |
| Laboratory tests | ||||
| MAP | 0.226 | 0.988 | 0.970 | 1.007 |
| FBG | 0.732 | 0.954 | 0.726 | 1.253 |
| Glycated hemoglobin | 0.690 | 1.036 | 0.870 | 1.234 |
| Scr | 0.239 | 0.988 | 0.968 | 1.008 |
| SUA | 0.021 | 1.005 | 1.001 | 1.009 |
| cTnI | 0.790 | 0.094 | 0.000 | ‐ |
| NT‐proBNP | 0.968 | 1.119 | 0.004 | 284.324 |
| TG | 0.215 | 1.260 | 0.875 | 1.815 |
| TC | 0.134 | 1.287 | 0.925 | 1.791 |
| LDL‐C | 0.011 | 1.977 | 1.167 | 3.347 |
| HDL‐C | 0.179 | 2.273 | 0.685 | 7.540 |
| Hs‐CRP | <0.001 | 2.160 | 1.711 | 2.726 |
| ESR | 0.070 | 0.921 | 0.843 | 1.007 |
| WBC | 0.316 | 0.889 | 0.707 | 1.119 |
| Neutrophil | 0.854 | 1.031 | 0.744 | 1.429 |
| Coronary angiography findings | ||||
| Multivessel artery lesions | 0.199 | 1.730 | 0.750 | 3.990 |
| Target lesion at LAD | 0.551 | 1.226 | 0.628 | 2.393 |
| Target lesion at LCX | 0.528 | 0.800 | 0.400 | 1.600 |
| Target lesion at RCA | 0.936 | 1.029 | 0.517 | 2.046 |
| Patients with two target lesions | 0.949 | 1.024 | 0.495 | 2.117 |
| Stenosis degree of target lesion | 0.491 | 1.020 | 0.963 | 1.081 |
| Length of target lesion | 0.032 | 1.043 | 1.004 | 1.085 |
| PCI surgery parameters | ||||
| Bifurcation requiring double wiring | 0.003 | 2.799 | 1.427 | 5.488 |
| Length of stent | 0.031 | 1.044 | 1.004 | 1.085 |
| Diameter of stent | 0.316 | 1.725 | 0.594 | 5.006 |
| Time of stent dilation | 0.354 | 0.962 | 0.885 | 1.045 |
| Balloon dilation pre‐stent | 0.593 | 1.208 | 0.605 | 2.411 |
| Drugs used after surgery | ||||
| Aspirin | ‐ | ‐ | ‐ | ‐ |
| Nitrates | 0.362 | 0.515 | 0.124 | 2.147 |
| Statins | 0.460 | 0.521 | 0.092 | 2.941 |
| β‐receptor blockers | 0.465 | 0.667 | 0.225 | 1.980 |
| ACEIs/ARBs | 0.867 | 1.064 | 0.516 | 2.193 |
| Calcium channel blockers | 0.666 | 1.162 | 0.588 | 2.296 |
BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; FBG, fasting blood‐glucose; Scr, serum creatinine; SUA, seru‑m uric acid; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; TG, triglyceride; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; LAD, left anterior descending branch; LCX, left circumflex artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; ACEIs/ARBs, angiotensin converting enzymes inhibitors/angiotensin receptor blockers.
Data were presented as P value, OR (odds ratio), and 95% CI (confidence interval). P value <0.05 was considered significant. Statistically significant values were shown in bold font. Factors affecting restenosis occurrence were determined by univariate logistic regression model analysis, and “‐” indicated that there was no statistical performance due to lack of events.
Table 7.
Factors affecting restenosis occurrence by multivariate logistic regression model analysis with the Forward Stepwise (Conditional) method
| Parameters | Multivariate logistic regression | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| DM | <0.001 | 23.721 | 5.636 | 99.842 |
| SUA | 0.049 | 1.006 | 1.000 | 1.013 |
| LDL‐C | <0.001 | 5.666 | 2.175 | 14.760 |
| Hs‐CRP | <0.001 | 3.143 | 2.114 | 4.674 |
| Length of target lesion | 0.002 | 1.124 | 1.044 | 1.211 |
| Bifurcation requiring double wiring | 0.009 | 4.645 | 1.458 | 14.800 |
DM, diabetes mellitus; SUA, serum uric acid; LDL‐C, low‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein.
Data were presented as P value, OR (odds ratio), and 95% CI (confidence interval). P value <0.05 was considered significant. Factors affecting restenosis occurrence were determined by multivariate logistic regression model analysis with the Forward Stepwise (Conditional) method. Statistically significant values were shown in bold font.
4. DISCUSSION
Our study discovered that in CAD patients underwent PCI with drug‐eluting stents implantation: (a) 1‐year occurrence of RASP was 37.8%, and DM, SUA, hs‐CRP, multivessel artery lesions as well as bifurcation requiring double wiring independently predicted elevated risk of RASP; (b) 1‐year occurrence of restenosis was 21.0%, and DM, SUA, LDL‐C, hs‐CRP, length of target lesion as well as bifurcation requiring double wiring independently predicted raised risk of restenosis.
In CAD treatment, the adoption of PCI and drug‐eluting stents presents great effect on unchoking the stenosis of coronary artery, preventing vessel wall recoil, and inhibiting neointimal growth of the targeted lesion, while the treatment efficacy has been frequently reported to be attenuated by occurrence of RASP and restenosis, which becomes one of the major clinical challenges for CAD treatment.16 Although the exact mechanism of RASP in CAD patients is unclear, it is currently accepted that rupture of plaques and the subsequent injury response facilitate the accretion of the vascular wall and contribute to RASP.17 Clinical studies disclose that CRP level is positively associated with RASP of nontarget lesion in angina patients after stents implantation.12, 13 Another study reports that elevation of CRP predicts rapid angiographic progression of nonculprit lesion in non‐ST‐segment elevation acute syndrome patients underwent PCI.17 These previous evidences reveal the role of CRP in predicting RASP risk in different types of CAD patients, while other possible factors are less investigated. In the present study, we collected comprehensive baseline, procedural, and postprocedure characteristics and discovered that DM, SUA, hs‐CRP, multivessel artery lesions, and bifurcation requiring double wiring independently predicted high risk of RASP. The possible explanations were (a) Patients with DM might be liable to develop atherosclerosis due to limited glycemic control, dyslipidemia as well as poor immunologic function, thereby exposing to higher risk of RASP. (b) High concentration of SUA might facilitate with the deposition of urate crystal at the vessel wall to cause vessel wall injury and promote inflammation, hence devoting to RASP in CAD patients.18 (c) Hs‐CRP might enhance the instability of plaques to induce plaque rupture, thereby contributing to increased risk of RASP.19 (d) Patients with multivessel artery lesions and bifurcation might be more vulnerable to plaque rupture, which raised RASP risk.
From literature, the incidences of restenosis are diverse in different studies. A study from Japan concludes that incidence of restenosis at ostial lesion of right coronary artery is 34% in 6‐18 months.20 In CAD patients complicated with DM, the incidence of restenosis is around 20% over 6 months after drug‐eluting stent implantation.3, 21 Whereas in our study, different follow‐up time (12 months), general CAD patients rather than CAD patients with specific complications were recruited, therefore, our study yielded different but reasonable incidence rate of restenosis being 21.0%. Restenosis is believed to be caused by arterial remodeling and neointimal hyperplasia, and plenty of studies have been performed investigating the factors associated with restenosis risk in CAD patients.6 DM history has been exhibited to be correlated with higher incidence of restenosis in CAD patients after PCI.22 And serum very low‐density lipoprotein cholesterol (VLDL‐C), SUA level, as well as history of PCI in CAD patients independently predict high restenosis risk.3 Also, elevated hs‐CRP level has been disclosed to promote the rate of restenosis in CAD patients after drug‐eluting stents implantation.19, 23 In line with these previous studies, we observed that DM, SUA, hs‐CRP, LDL‐C, length of target lesion, and bifurcation requiring double wiring independently predicted increased restenosis risk. This might due to that: (a) CAD patients complicated with DM had specific angiographic characteristics including smaller vessel dimensions and longer lesions, thereby increasing the susceptibility to restenosis.21 (b) Raised SUA level might boost the secretion of inflammatory cytokines, platelet‐derived growth factor, or angiotensin to promote the proliferation of vascular smooth muscle cells and consequently leading to neointimal hyperplasia as well as increased risk of restenosis.18 (c) In response to acute injury, hs‐CRP was elevated with enhanced inflammation at the stented sites, increasing the proliferation of intima cells, thereby promoting restenosis. (d) Oxidized LDL‐C deposition was the main cause for initiation of atherosclerosis and a crucial risk factor for stenosis in coronary arteries, therefore, elevated LDL‐C might devote to high occurrence of restenosis as well.24, 25 (e) For the target lesion, the injured area in intima was increased with the length of target lesion, to raise the risk of neointimal hyperplasia in coronary arteries, thereby accelerating restenosis in the stents. (f) The procedure for bifurcation that required double wiring might result in overlapping of stents and improper expansion of stents, which might alter treatment efficacy and increase the risk of restenosis.
There were still several limitations in our study. Firstly, RASP and restenosis were evaluated at 12 months after PCI; thus, the correlation of the candidate factors with the long‐term RASP and restenosis risk in CAD patients underwent PCI with drug‐eluting stents implantation was not investigated. Secondly, the number of patients recruited was relatively small, and large scale of predictive factors was included for analysis, which might reduce the statistical power. Thirdly, a large number of variables that were possible influential factors were included for analysis at significant level of 0.05, which was at risk of yielding meaningful result by accident.
In conclusion, DM, SUA, and hs‐CRP are potential predictive factors for increased risk of both RASP and restenosis in CAD patients underwent PCI and drug‐eluting stents implantation.
Wu Y, Fu X. Comprehensive analysis of predictive factors for rapid angiographic stenotic progression and restenosis risk in coronary artery disease patients underwent percutaneous coronary intervention with drug‐eluting stents implantation. J Clin Lab Anal. 2019;33:e22666 10.1002/jcla.22666
REFERENCES
- 1. Assimes TL, Roberts R. Genetics: implications for prevention and management of coronary artery disease. J Am Coll Cardiol. 2016;68:2797‐2818. [DOI] [PubMed] [Google Scholar]
- 2. Trikalinos TA, Alsheikh‐Ali AA, Tatsioni A, et al. Percutaneous coronary interventions for non‐acute coronary artery disease: a quantitative 20‐year synopsis and a network meta‐analysis. Lancet. 2009;373:911‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang JL, Qin Z, Wang ZJ, et al. New predictors of in‐stent restenosis in patients with diabetes mellitus undergoing percutaneous coronary intervention with drug‐eluting stent. J Geriatr Cardiol. 2018;15:137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoki J, Kirtane A, Leon MB, et al. Coronary artery aneurysms after drug‐eluting stent implantation. JACC Cardiovasc Interv. 2008;1:14‐21. [DOI] [PubMed] [Google Scholar]
- 5. Cui K, Lyu S, Song X, et al. Drug‐eluting balloon versus bare‐mental stent and drug‐eluting stent for de novo coronary artery disease: a systematic review and meta‐analysis of 14 randomized controlled trials. PLoS ONE. 2017;12:e0176365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Her AY, Shin ES. Current management of in‐stent restenosis. Korean Circ J. 2018;48:337‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sukhija R, Aronow WS, Sureddi R, et al. Predictors of in‐stent restenosis and patient outcome after percutaneous coronary intervention in patients with diabetes mellitus. Am J Cardiol. 2007;100:777‐780. [DOI] [PubMed] [Google Scholar]
- 9. Zhao L, Zhu W, Zhang X, et al. Effect of diabetes mellitus on long‐term outcomes after repeat drug‐eluting stent implantation for in‐stent restenosis. BMC Cardiovasc Disord. 2017;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kornowski R, Hong MK, Tio FO, et al. In‐stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224‐230. [DOI] [PubMed] [Google Scholar]
- 11. Gogo PB Jr, Schneider DJ, Watkins MW, et al. Systemic inflammation after drug‐eluting stent placement. J Thromb Thrombolysis. 2005;19:87‐92. [DOI] [PubMed] [Google Scholar]
- 12. Xu YL, Li JJ, Xu B, et al. Increased plasma C‐reactive protein level predicts rapid progression of non‐target atherosclerotic lesions in patients with stable angina after stenting. Chin Med J (Engl). 2011;124:3022‐3029. [PubMed] [Google Scholar]
- 13. Imai K, Okura H, Kume T, et al. C‐reactive protein predicts non‐target lesion revascularization and cardiac events following percutaneous coronary intervention in patients with angina pectoris. J Cardiol. 2009;53:388‐395. [DOI] [PubMed] [Google Scholar]
- 14. Lubrano V, Balzan S. Consolidated and emerging inflammatory markers in coronary artery disease. World J Exp Med. 2015;5:21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaubert M, Marlinge M, Alessandrini M, et al. Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signal. 2018;14:191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalal F, Dalal HM, Voukalis C, et al. Management of patients after primary percutaneous coronary intervention for myocardial infarction. BMJ. 2017;358:j3237. [DOI] [PubMed] [Google Scholar]
- 17. Nakachi T, Kosuge M, Hibi K, et al. C‐reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non‐ST‐segment elevation acute coronary syndrome. Circ J. 2008;72:1953‐1959. [DOI] [PubMed] [Google Scholar]
- 18. Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and coronary heart disease: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken). 2010;62:170‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zairis MN, Manousakis SJ, Stefanidis AS, et al. C‐reactive protein and rapidly progressive coronary artery disease–is there any relation? Clin Cardiol. 2003;26:85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watanabe Y, Takagi K, Naganuma T, et al. Independent predictors of in‐stent restenosis after drug‐eluting stent implantation for ostial right coronary artery lesions. Int J Cardiol. 2017;240:108‐113. [DOI] [PubMed] [Google Scholar]
- 21. Radke PW, Friese K, Buhr A, et al. Comparison of coronary restenosis rates in matched patients with versus without diabetes mellitus. Am J Cardiol. 2006;98:1218‐1222. [DOI] [PubMed] [Google Scholar]
- 22. Gilbert J, Raboud J, Zinman B. Meta‐analysis of the effect of diabetes on restenosis rates among patients receiving coronary angioplasty stenting. Diabetes Care. 2004;27:990‐994. [DOI] [PubMed] [Google Scholar]
- 23. Hsieh IC, Chen CC, Hsieh MJ, et al. Prognostic impact of 9‐month high‐sensitivity C‐reactive protein levels on long‐term clinical outcomes and in‐stent restenosis in patients at 9 months after drug‐eluting stent implantation. PLoS ONE. 2015;10:e0138512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lusis AJ. Atherosclerosis. Nature. 2000;407:233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503‐516. [DOI] [PubMed] [Google Scholar]


